To Issue 133
Citation: Eden O, “Connected Drug Delivery – Maximising Access by Design”. ONdrugDelivery, Issue 133 (May 2022), pp 81–84.
Oliver Eden discusses the factors that drive the design and development of a reusable autoinjector solution. Foremost amongst these considerations is the patient, in terms of ease of use with the device itself and maximising access to the broadest possible population with a versatile, portfolio-enabling platform ready for the connected world.
“You’ve got to start with the customer experience and work back toward the technology – not the other way around.” Apple founder Steve Jobs spoke these oft-quoted words as part of a Q&A at the company’s 1997 Worldwide Developer Conference. It’s an insightful statement but can be very challenging to do in healthcare, where an interplay of core objectives – access, quality and cost containment – can sometimes lead to conflicting priorities among the many different participants in its ecosystem.
Healthcare practitioners (HCPs) strive to improve patients’ lives, regulators work towards a balance between clinical outcome and safety, whilst payers and insurance companies work to balance clinical outcome and total healthcare costs. At the same time, pharmaceutical companies seek approval and reimbursement for therapies that will be clinically successful and commercially viable. And, at the centre of it all, is the patient, seeking improved health with access to effective treatments that are as minimally disruptive to their everyday life as possible.
The key to good design for developing or improving a medical device, such as an autoinjector, is to walk in each of these stakeholders’ footsteps to understand their combined perspectives, goals and challenges, and find an ideal balance between functionality, usability and cost that will benefit them all in their mutual pursuit of improving patient outcomes.
“Optimising the device’s design to secure the greatest advantage for the largest potential user population is an acceptable and strategically sound compromise.”
A MANUFACTURER’S PERSPECTIVE
When it comes to strategic ways to address the future needs of Jabil’s customers, meaningful insights come when the team asks the right questions. What are the technical and business process opportunities that will deliver value to customers and the broader healthcare ecosystem – the patients and their care teams, the payers and providers?
The goal is to develop a partnership that goes beyond simply matching requirements with capabilities – the answer to “How do we build it?” – towards a more complete– how to build it simply, more sustainably, with fewer supply chain nodes and a lower bill of materials, and modularly so that a product is easily upgradeable as a hedge to changing market dynamics.

Figure 1: Industry trends driving innovation.
How can sound product lifecycle management principles, combined with design for manufacturability (DfM) expertise, inform the development of an autoinjector device platform – whilst also aligning the solution with powerful, emergent trends in pharma delivery. Such trends include advancements in biologics; the shift to more patient self-care in the home; demand for connected, digital platform enhancements; and increasing calls to improve medical device sustainability metrics (Figure 1).
QFINITY™ ANSWERS THE CALL
Qfinity is a platform for subcutaneous (SC) drug self-administration, centred on a reusable autoinjector that supports the emerging prioritisation within pharma for more sustainable drug delivery (Figure 2). Designed to help pharma customers get to market quickly, at a lower cost, Qfinity accommodates the direction of travel in portfolio characteristics, both in terms of formulation properties (e.g. viscosity) and delivery volumes.
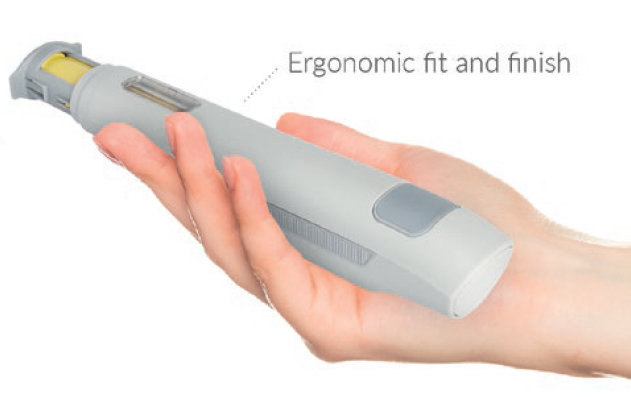
Figure 2: The Qfinity autoinjector.
Additionally, the system provides a low-cost pathway to digital health connectivity for all players in the healthcare ecosystem with Qfinity+™. This connected version features a home hub that provides wireless charging and seamless cellular data transfer functionality in near-real time (Figure 3), without requiring any change in drive unit form factor between the two versions – the non-connected solution, Qfinity, and the connected solution, Qfinity+.

Figure 3: The Qfinity cellular home hub.
At the centre of the platform is a simple spring-loaded reusable autoinjector with a single form factor drive unit. The use of a mechanical drive system is fit for function, negating the requirement for an alternative drive system (e.g. electromechanical) that would add unnecessary cost without significantly improving function. The drive device accommodates and operates with either of two disposable cassettes – one for each of 1 mL and 2.25 mL prefilled syringes (PFSs).
Purposefully designed to be versatile and portfolio enabling, Qfinity provides a path to connected healthcare without added complexity or additional cost in a more sustainable, competitively priced solution for use in both clinical development and commercial supply.
“Capture and transmission of data is delivered seamlessly by virtue of the home hub solution, which accommodates both charging and data transfer without requiring input from the patient.”
ADVANTAGES FOR PATIENTS
Ongoing human factors studies bear out high marks for ease of use per Qfinity’s form factor, which is exactly the same for both the connected and non-connected versions, as is the user operation sequence. Precedented form factor and feature sets are well considered and validate design choices, requiring no retraining. Users report that the drive device “feels right” in terms of its shape and that it is intuitive and “easy to use”.
For users accustomed to disposable injectors, the additional step of loading a cassette is also reported as being simple and straightforward. Inclusive design principles and usability engineering have proven effective in securing Qfinity’s developers’ objectives for an easy-to-use, easy-to-teach disposable cassette and reusable injector experience.
Assessments bear out the inevitable trade-offs inherent in medical device design. Sustainability and other value advantages offered by a reusable device impact an autoinjector’s overall size and shape. In some instances, devices that are too small or compact can be detrimental in terms of grip function. For example, as demonstrated by users with rheumatoid arthritis, a larger-diameter device is likely to be easier for them to close their hand around.
In the context of standard use for these types of products – typically at-home self-administration no more than once weekly – optimising the device’s design to secure the greatest advantage for the largest potential user population is an acceptable and strategically sound compromise.
THE PLUS IN QFINITY+
As for the connected version, Qfinity+ allows medical teams to remotely monitor their patients’ care and compliance via built-in sensors and electronics. This does not increase the version’s complexity, as the device’s drive unit form factor and the use steps are exactly the same as the non-connected version. Capture and transmission of data is delivered seamlessly by virtue of the home hub solution, which accommodates both charging and data transfer without requiring input from the patient. Qfinity+ delivers this improved adherence benefit – seamless, real-time event tracking for accurately measuring compliance – at a cost 20% lower than current market-leading non-connected disposable autoinjectors.
DEEPER DIVE ON THE QFINITY+ CONNECTIVITY SOLUTION
Within the disposable mechanical autoinjector market, a common route for delivering connectivity is a “sleeve” that fits over the autoinjector and captures adherence and/or compliance data and communicates it to the user’s smartphone via Bluetooth Low Energy (BLE) protocols. Jabil’s decision to design a cellular home hub solution for Qfinity+ instead goes back to the company’s primary objective: maximise access.
The sleeve solution adds user steps and complicates a patient’s therapy rather than simplifying it. Sleeves require attaching and detaching, pairing the injector device with a smartphone, downloading and maintaining an app and co-locating the injector and the smartphone. There are several assumptions inherent in this model, for example that the patient has some technical know-how and can learn how to perform the necessary steps. Of course, a patient must also have a smartphone in the first place!
For broad populations in the developing world, as well as in more advanced markets, ownership of smartphones is not guaranteed. Nor is the physical ability to manage these variables something that can be assured in what are often older or infirm patient populations.
Analysis of smartphone ownership by age, socio-economic status and level of education provides critical insight. Even in a technologically advanced country like the US, smartphone ownership amongst people over 65 years old – a key demographic for healthcare – is only 61% (Figure 4). Throughout the developed and developing world, coverage gaps exist and must be considered. The home hub for Qfinity+ was conceived to negate the accessibility issues inherent in requiring a smartphone, as its connectivity solution is based on cellular communications coverage, which is typically greater than 95% worldwide and delivered seamlessly without the requirement for any additional devices.
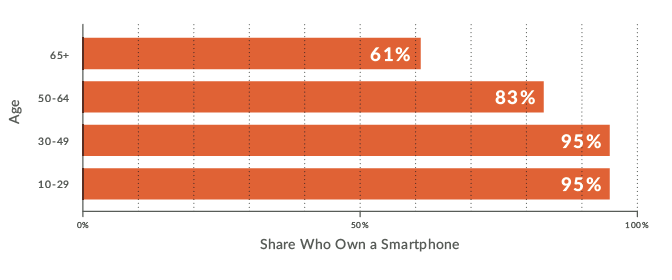
Figure 4: Smartphone ownership by age.
“Qfinity’s reusable mechanical drive unit consists of 19 components and the disposable cassette assembly consists of only four moulded components, plus the PFS.”
BENEFITS WITHIN CLINICAL DEVELOPMENT
Within clinical development, the integration of digital technology is transforming how studies are conducted. However, all the benefits connectivity provides for personalising and strengthening patient engagement for the emerging practice of decentralised trials are dependent on trial participants actually using their device’s connectivity features.
Communication platforms dependent on a study participant having access to a smartphone means that either pharma companies need to provide a smartphone for the participants or listing a smartphone as a criterion for participation in the study. Both options are challenging for studies conducted in regions with low smartphone ownership (Figure 5), and the latter option is simply not acceptable, as it would bias the study population and potentially impact study timelines.
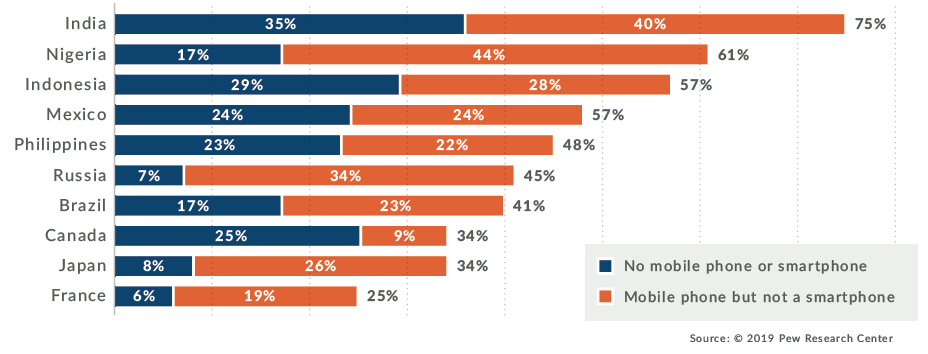
Figure 5: Smartphone and mobile phone ownership by region.
The combined design requirements of Qfinity+, revolving around access, quality and cost, provided a clear challenge for its designers – one which catalysed the team’s inspiration for the home hub. The resulting solution became another emblem of versatility-driven design, enabling both connectivity and charging by virtue of more universal cellular communications, being independent of smartphones, and requiring no additional user steps or training for a truly patient-centric product platform. Data is captured seamlessly without requiring action from the patient, potentially driving better adherence by simplifying correct usage and enabling more proactive management of studies by their sponsors.
REUSABLE IS MORE SUSTAINABLE
One of the human factors participants stated things quite simply: “Sustainability affects us all.” Another said: “You’re doing something to stay healthy and then also making your planet healthy.”
The disposable autoinjectors available today have several different form factors and share very few components. From a pharma company perspective, while these simple mechanical solutions are portfolio enabling, they increase manufacturing footprint requirements, drive additional capital expenditure, result in more medical waste and increase supply chain complexity – all of which have an impact on the cost of goods produced.
Qfinity’s reusable mechanical drive unit consists of 19 components and the disposable cassette assembly consists of only four moulded components (plus the PFS). The Qfinity 1 mL and 2.25 mL design variants share up to 80% of their components in a common form factor. In contrast, the market-leading disposable 1 mL and 2.25 mL disposable autoinjectors both feature 15–20 components, and then the device is only used once before being thrown away.
In other words, Qfinity delivers the equivalent portfolio-enabling functionality as the market-leading 1 mL and 2.25 mL disposable autoinjectors combined at a lower cost of goods and in a more sustainable solution.
Jabil’s designers conducted a lifecycle assessment to determine the cost benefits captured within Qfinity’s platform design, with a particular focus on the benefits of the reusable drive unit. Due in large part to the fact that the design requires less material, it is estimated that Qfinity delivers a 65% reduction in cost per injection compared with market-leading disposable autoinjectors, as well as delivering improved sustainability metrics (Figure 6).
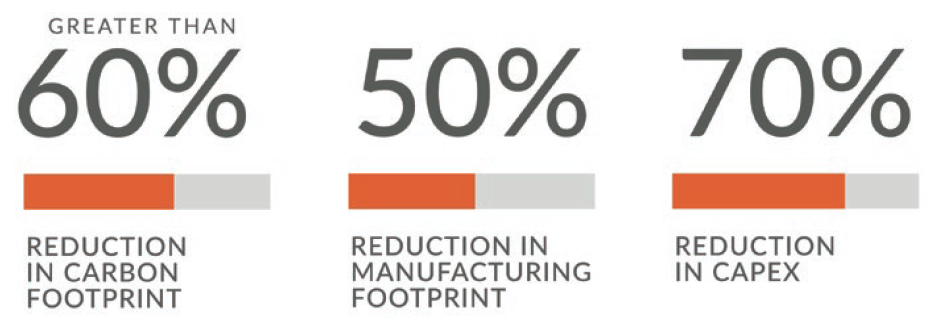
Figure 6: Sustainability improvements delivered by Qfinity compared with market-leading disposable autoinjectors.
This assessment considered the carbon footprint from sourcing, manufacturing and supply to a pharma partner but did not take into account the savings in transportation afforded by the greater packaging density possible due to Qfinity’s compact cassette or cold chain storage costs.
QFINITY – INSIGHT DELIVERED
Qfinity comes to market as one of the most versatile autoinjector platforms available today. Sustainable, accessible and inclusive, both in terms of ease of use and the seamless connectivity option it provides for enhanced digital health, Qfinity is deftly positioned at the intersection of converging value trends within pharma. Accommodating the fullest range of requirements from across the healthcare ecosystem, Qfinity is a simple and insightful solution to meet the industry’s current and future needs.
Previous article
ADVANCEMENTS AND EVOLUTION OF POWER SOURCES IN DRUG DELIVERYNext article
INJECTING THE FUTURE
