To Issue 156
Citation: Buchine B, Durkin J, “Designing for Success: Choosing the Right Primary Drug Container for Prefilled Delivery Devices”. ONdrugDelivery, Issue 156 (Jan 2024), pp 46–50.
Brent Buchine and Jason Durkin reflect on the motivations and lessons from Windgap Medical’s journey to design patient-centric, prefilled autoinjectors around custom and standard primary drug containers.
As a company founded to simplify, automate and accelerate the administration of complex drug therapies, Windgap often finds itself asking an important question when approaching a new development programme:
Do we innovate around a standard primary drug container (PDC) with all its existing benefits and limitations – or do we create a novel solution to better address the needs of the target patient population?
The answer, of course, is almost as complex as the drugs themselves. In this article, Windgap shares the possibilities – and problems – associated with each approach and sheds light on new opportunities that are forming the frontier of pharma.
“In some cases, the standard primary drug container is perfectly suitable; in others, it must be completely reimagined to support a combination product that suits the needs of the patient, the drug requirements and the market demands.”
A SHIFTING DECISION MATRIX
When initiating a programme to design and develop an injection device for drug delivery, one of the first major decisions is the selection of the PDC. Various standard options are available, including the prefilled syringe (PFS), cartridge and vial, as well as the mated plungers, cannulas, sterility barriers, seals and other accessories that make up the complete PDC system.
Several considerations drive the decisions behind these options – device type (e.g. pen injector, autoinjector, on-body wearable, etc), whether the device will be disposable or reusable, and how it interacts with the drug product. In the past, these considerations alone could lead to a clear decision, especially as indications often have a preferred PDC format with widespread industry adoption.
With today’s injectable therapies, however, increasingly complex molecules and value chains require additional evaluation. Taking the disposable autoinjector as an example, therapy trends that preclude the conventional use of a standard 1 or 2.25 mL PFS include:
- The use of lyophilisation to enhance stability, particularly for high-value monoclonal antibodies and biologics
- Long-acting injectables, formulated as a nanoparticle suspension, require separation from the liquid vehicle until mixing and administration
- Co-administration of two drugs with formulation and storage incompatibilities.
The administration challenges of these drug products are compounded further when also incorporating the needs of human factors, high-viscosity and large-volume dosage requirements, and a strong desire to stay as close to the conventional “two-step” autoinjector as possible. In some cases, the standard primary drug container is perfectly suitable; in others, it must be completely reimagined to support a combination product that suits the needs of the patient, the drug requirements and the market demands.
As a company known for its focus on “injecting simplicity” into complex drug delivery, Windgap faced this dilemma when developing each of its current product platforms. Its large-volume, dual-chamber (LVDC) device relies on proven cartridges, while the ANDI® device was intentionally developed around a completely custom PDC design. With each development journey, Windgap established a deep understanding of the risks and benefits of either pathway, giving the company an intimate appreciation of when and why to do either.
SETTING THE STAGE: STANDARD VERSUS CUSTOM
From supply constraints to added capital costs, standard and custom approaches each bring pros and cons that must be carefully assessed before beginning the long journey of product design and development. These considerations are described below and summarised in Table 1.
| Standard PDC | Custom PDC | |
| Benefits |
|
|
| Challenges |
|
|
Table 1: Summary analysis of standard versus custom PDC decision matrix.
“Leveraging a proprietary needle hub and regulated gas power, this platform uses two single-chamber cartridges to administer therapies requiring reconstitution, liquid/liquid mixing or sequential delivery.”
The “Standard” Scenario
A standard primary container typically consists of a glass syringe with a cannula and elastomer plunger or a glass cartridge with an elastomer seal and plunger; less commonly, cyclo-olefin polymer or copolymer (COP/COC) may be used in place of glass. Several world-class suppliers of these components have become the norm for injection systems over time. Today, off-the-shelf components provide a low-risk, cost-effective option with a long history of manufacturing optimisation and use on common filling lines.
While mature and known, the manufacturing of standard glass primary containers has recently been plagued by demand spikes, supply constraints and long lead times. In addition, standard containers are nearly always coated with silicone to reduce glide forces and PFSs may contain traces of tungsten from the fluid path-forming process – both of which can lead to stability and particulate issues for the contained drug.1
“Custom” Considerations
COP is often the material of choice for a custom development project. Custom injection-moulded primary containers offer the benefit of bespoke shapes and sizes alongside additional functionality, for example, assembly features or an insert-moulded cannula. In some cases, the use of silicone can be avoided for drugs with which it is not compatible. This is due to the reduced friction between a plunger and polymeric barrel as compared with glass or the use of dry film lubricants.2
Despite the advantages of polymeric materials and custom manufacturing, these custom containers introduce new challenges not typically seen with standard PDCs. Multiple suppliers must be identified and managed for product design. A custom filling line may be required, surface-drug interactions must be characterised to ensure drug compatibility and non-glass containers may threaten drug stability due to increased gas permeation. Properly addressing such risks may result in a longer development cycle and require significant capital investment.
THE JOURNEY TO DESIGNING A FULLY CUSTOM PDC
Early in Windgap’s development programme for emergency injections with its ANDI® device, the company found the widely available standardised systems forced it to sacrifice the patient experience and depart from its ideal target product profile. Windgap faced constant trade-offs between device form factor, usability, performance and other attributes. To satisfy all user needs, the company began to explore design options relying on a novel PDC architecture that would resolve these dilemmas.
The final design (Figure 1) achieved the integration of all critical functions while maintaining a compact, portable form factor.
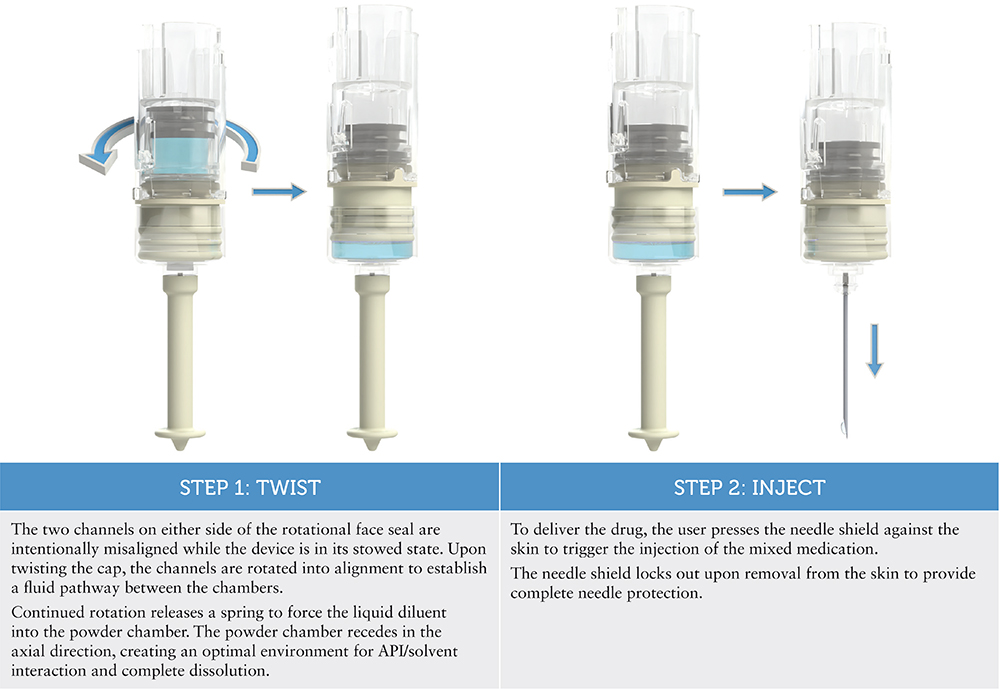
Figure 1: The ANDI® device, a revolutionary “twist” on emergency autoinjectors, administers injections in just two steps thanks to its custom PDC design.
The Advantages of Innovative Design
Windgap takes “injecting simplicity” seriously – the mixing mechanism for the ANDI® device is driven mechanically by the user, who simply twists the cap for removal. This action automatically rehydrates the powdered drug in just a few seconds, thanks to design-controlled fluidics – no shaking or swirling necessary.
The key component of the custom PDC design is a rotating face seal between the powder and liquid chambers and how the twisting user input is connected to it. Windgap was unable to identify anything “off the shelf” that would have been able to achieve the level of simplicity that this device offers while still being small enough to carry comfortably.
Unexpected Challenges – and Custom Solutions
The implications of Windgap’s decision to move forward with a custom PDC affected both budget and timeline. Given the novelty of the PDC, the company had to develop a compatible filling method rather than relying on existing standard equipment and processes. It also had to ensure the entire supply chain and overarching quality management system complied with 21 CFR 210 and 211 requirements for drug-device combination product manufacturers and included all systems required for properly managing drug handling. On the design side, Windgap balanced its sterilisation strategy with efforts to achieve acceptable container closure integrity and seal integrity.
Despite the array of challenges, the decision to pursue a fully customised PDC remained appropriate as it offered several significant benefits along the way:
- Windgap could hold tight tolerances on critical parts specific to its design
- Given its control over the filling process, the company eliminated the risk of variable lead times with contract manufacturers as it scheduled filling runs
- Windgap was able to navigate the covid-19-related supply chain disruptions in glass sourcing and ongoing capacity constraints from the rise of GLP-1 volumes, thanks to its independently managed network of component suppliers
- The company maintained best-in-class performance requirements to deliver effective and efficient product attributes to patients.

Figure 2: Putting standard cartridges to work in new ways with Windgap’s LVDC side-by-side dual-chamber architecture, in this example for the reconstitution products.
LEVERAGING STANDARD PDCs IN A NEW WAY
With ANDI® well positioned for commercial development through a product partnership with ALK-Abelló (Hørsholm, Denmark),3 Windgap set out to develop another device platform around a new set of requirements inspired by continued customer engagement. One requirement was compatibility with readily available, proven glass cartridges.
The resulting LVDC platform reimagines the dual-chamber drug delivery system by maintaining the usability and aesthetics of a conventional, single-chamber autoinjector. Leveraging a proprietary needle hub and regulated gas power, this platform uses two single-chamber cartridges to administer therapies requiring reconstitution, liquid/liquid mixing or sequential delivery. This innovative arrangement of off-the-shelf PDCs substantially streamlines the administration process for these complex formulations to just three steps, as shown in Figure 2.
Elevating the Standard Solution with the Patient in Mind
The LVDC’s nested PDC architecture accommodates the use of two readily available, ISO-compliant cartridges (from 1 to 5 mL) compatible with industry-standard fill-finish processes while maintaining a compact, easy-to-handle form factor.
LVDC products are gas powered to enhance functionality when managing both high-viscosity and large-volume injections. For reconstitution or liquid/liquid mixing applications, this removes the need to shake or swirl, substantially reducing the steps required for prep and administration. Users can activate and regulate the mixing and administration of complex and combined drug therapies with a press of a single button.
When the mixing device variant is in its fully automatic configuration, treatment may be delivered in just three steps – initiate mixing, remove cap, inject – to minimise user effort and required training.
Furthermore, the controlled, reciprocated mixing between the side-by-side cartridges presents an exciting opportunity to shift from manual, subjective mixing to device-controlled, validated mixing. Whether reconstituting a dry powder or mixing two liquids, such a shift promises more consistent mixing outcomes and reduces the risk of error from instructions to “shake”, “swirl” or “tap” at the point of care. This is just one example of how Windgap empowers patients by designing with the end use – and the end user – in mind.
TURNING NOVELTY INTO THE NEW NARRATIVE
Windgap has accumulated experience developing injectable combination products with both custom and standard, off-the shelf PDCs and as part of a novel combination product. This knowledge is used to inform the company’s collaborations with biopharmaceutical partners to bring together device and drug or biologic into a robust combination product.
Windgap encourages its fellow device innovators and pharmaceutical counterparts to consider the following:
- If combination product requirements can be met with a standard off-the-shelf PDC system, this option should be prioritised, especially if the intention is to develop an injector platform that would be supplied to other pharmaceutical companies, which are historically risk-averse.
- If developing a full combination product in-house, there is more latitude and innovation freedom for custom designs to improve performance. Such customisation brings increased risk, investment and development time. However, if companies are willing and able to accept the challenge of developing and integrating non-standard aspects of the supply chain, such as drug handling and drug filling, the rewards can be substantial.
Windgap believes that the strongest and most critical innovations align the requirements of the drug, the needs of the patient, and the ability of the surrounding science and supply chains to meet both. Some statements are forward-looking. Unless specifically stated, these devices are not approved for sale in the US or the EU.
REFERENCES
- Murphy M et al, “Effect of Various Silicone Oil and Tungsten Levels on the Stability of a Monoclonal Antibody in Nine Commercially Available Prefilled Syringes”. J Pharm Sci, 2023, Vol 112(6), pp 1586–1594.
- Hlobik T, “Polymer Syringe Considerations for Drug Applications and Administration”. ONdrugDelivery, Issue 113 (Oct 2020), pp 90–93.
- Windgap Medical Announces Partnership with ALK-Abelló for its Epinephrine Autoinjector”. Press Release, Windgap Medical. Aug 9, 2019.
Previous article
CAPA VALVE’S PATENTED TECHNOLOGY TRANSFORMS ANY SYRINGE INTO A DCDNext article
PRACTICAL PATHWAYS TO SUSTAINABILITY
