Citation: Davidson Z, “Developing an Efficient Ophthalmic Device Combination Product”. ONdrugDelivery Magazine, Issue 104 (Jan 2020), pp 16-19.
Zoë Davidson looks at how understanding the patient journey can help develop an efficient ophthalmic device combination product.
A significant patient population suffers from conditions requiring long-term daily use of eye drops. Dry eye syndrome is associated with ageing, contact lens use and environmental factors. It affects an estimated 5% of over-50s in the US1 and is usually managed using an artificial tear solution which needs to be applied up to six times a day, often for the rest of the patient’s life. Other chronic conditions, such as glaucoma and hayfever, also require the long-term use of self-administered eye drops.
The majority of eye drops today contain preservatives to maintain the formulation’s sterility. The most commonly used preservative is benzalkonium chloride, which is known to damage the cornea over long-term use. Preservatives can also cause allergies or ocular irritation, and some can even cause a toxic response.2 Any such reactions are particular issues for patients who rely on the long-term use of eye drops for chronic conditions.
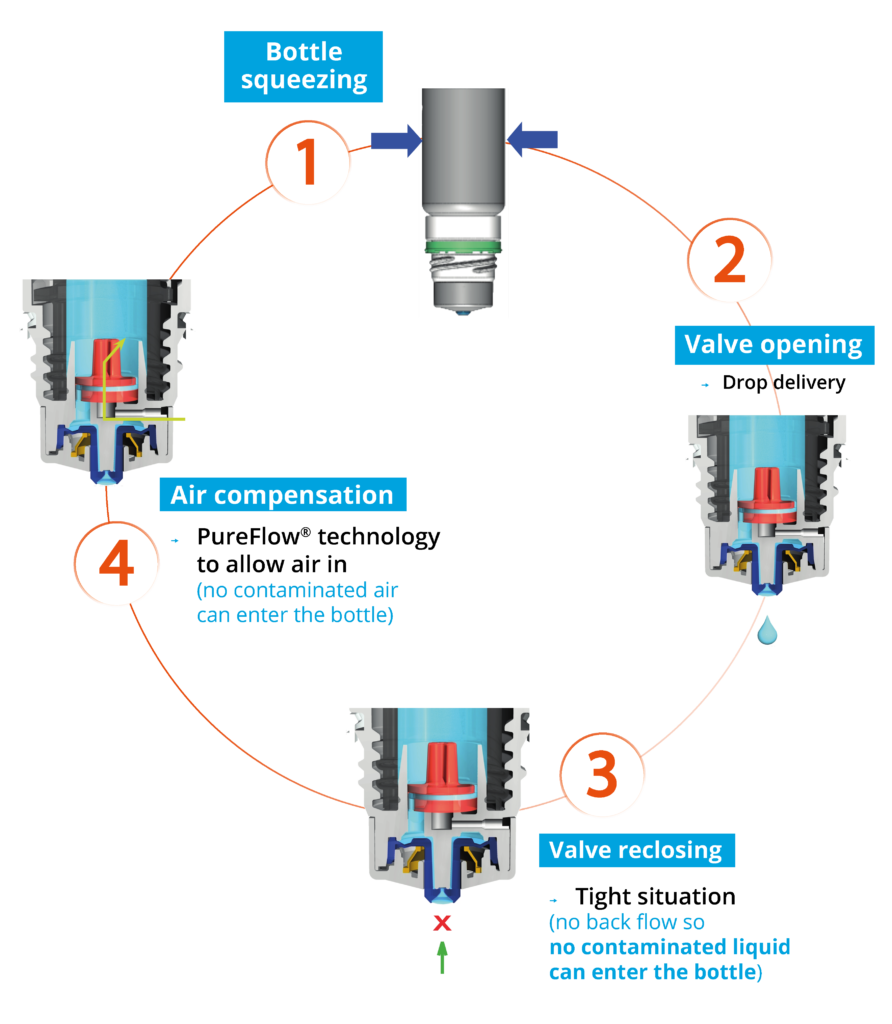
Figure 1: The Novelia system uses a non-return valve that removes the need to filter the liquid. This makes it possible to use a silicone membrane to filter the air.
THE POWER OF PUREFLOW TECHNOLOGY
To prevent the entry of bacteria into the bottle and/or to filter air, more than half of bottles designed for multi-dose preservative-free eye drops on the market rely on a filtering system – with 0.22 μm sterile mesh filters being the industry standard. But significant research has been carried out that challenges their effectiveness.3 Due to their porous structure, bacterial filters do not provide a continuous barrier to contamination.
Nemera’s alternative to the use of sterile filters for multi-dose preservative-free eye droppers is a non-return valve system used in conjunction with a silicone membrane to filter the returning air. The non-return valve ensures that no contaminated liquid can be re-introduced to the container after the drop has been dispensed – completely removing the need to filter the liquid. The intake of air into the Novelia dispenser takes place via a separate venting system with a silicone membrane called PureFlow Technology (Figure 1). The silicone membrane is a solid, non-porous (unlike bacterial filters) material. It is homogeneous and does not contain any holes – therefore its characteristics can be precisely engineered.
As well as serving as a venting system for air diffusion into the bottle, Novelia’s PureFlow has a second function: flow control. Nemera has adapted the flow-control technology within Novelia that avoids multiple drop delivery into the eye and ensures that only one calibrated drop is dispensed at a time. Nemera offers three different PureFlow versions, each tailored to formulations of differing viscosities, from highly liquid to highly viscous.
NOT JUST A PRODUCT BUT A PLATFORM
Novelia is not just one product but a platform, designed and developed with patients in mind. The demands of the ophthalmic market are increasingly varied in terms of new formulations and compositions. That’s why adaptability is a key criteria for Nemera.
“Effective drug delivery and patient adherence to treatments are increasingly important considerations in ophthalmology.”
Available to Novelia are five different valve sizes, each one delivering a different calibrated drop size. This allows Nemera’s team to customise the drop size depending on specific product requirements – a factor which is particularly important for generics companies where the generic product must replicate the same drop size, as well as the composition, of the originator product.
A range of bottles is available in terms of size, material and sterilisation type: 5 mL, 7.5 mL, 11 mL and 15 mL (Figure 2). All sizes are available in low-density polyethylene. The Nemera is also developing polypropylene and cyclic olefin copolymer bottles for specific formulation compatibility. Novelia has been validated using both gamma and ethylene oxide sterilisation. Offering two options for sterilisation allows Nemera to meet customers’ compatibility needs better.

Figure 2: Novelia has a full range of bottles available in terms of size, material and sterilisation type.
In addition to Novelia’s standard white cap colour, Nemera is able to develop additional colours for specific demands. Additional cap options include a vented cap, designed for sticky formulations, and a child-resistant cap.
NOVELIA PREFERRED BY 76% OF PATIENTS
“We put patients first” is not just a motto used by Nemera but an attitude firmly ingrained in the company’s culture. The earlier Nemera can include patients in the development phase to assess their behaviour versus new design, the better. Patients’ needs and constraints are generally identified when defining the product design brief and are included in Nemera’s quality process and documents such as product specification, design and user failure mode effects analysis.

Figure 3: 76% patients preferred Novelia compared with other preservative-free
multi-dose eye droppers on the market.
In 2015, Nemera had user tests carried out by an independent company.4 The panel of patients interviewed fell into two main criteria: demographics (age, gender) and type of eye condition (glaucoma, dry eye, etc.). These patients were interviewed in their own homes in both the US and the UK. These tests concluded that 76% of patients interviewed (68 out of 90 users) preferred Novelia over other similar devices on the market (Figure 3). Contributing factors to Novelia preference included the intuitiveness of the screw-on cap and the associated reassurance, and the squeeze force required towards the end of the product’s life. Novelia required only 6% more pressure to squeeze the bottle from the beginning to the end of the treatment, compared with 35% for the other device.
Originally intended to be transparent, Novelia’s patented blue tip was the result of a previous user study, where patients signalled that a coloured tip would help them target their eye. This feature was another aspect favoured by patients during the 2015 study.
SUPPORTING CUSTOMERS BY OFFERING A RANGE OF SERVICES
No single formulation is the same as another. Each has its own set of characteristics, such as viscosity or look (emulsion versus gel, for example), which impact its behaviour and delivery to the patient.
Nemera offers a range of laboratory services, including testing of customers’ bulk formulation. This testing comprises usage simulation over a two-week period, drop size analysis (variable depending on valve diameter), flow control and squeeze force testing (beginning and near end of life). The culmination of these tests results allows Nemera to determine the best Novelia configuration for a particular customer formulation. Nemera can recommend the most suitable PureFlow control, bottle type and valve size to achieve the desired drop calibration.
Nemera can also assist customers in finding the right ready-to-go dossier available for private labelling certain molecules with the Novelia device. Nemera has a list of partners, formulation licensors and fillers, all working in collaboration to bring customers a finished drug device combination with Novelia.
Finally, with more than 160 references on the market for prescription and over-the-counter products, Nemera’s regulatory team is on hand to support customers with their submission filing and provide guidance on supportive documents for registration.
CONNECTED DEVICES HAVE A ROLE IN HELPING PATIENTS WITH ADHERENCE

Figure 4: Nemera’s e-Novelia
ophthalmic add-on won the “Excellence
in Pharma: Drug Delivery Devices”
Award at CPhI Worldwide 2018.
In the case of chronic eye conditions such as glaucoma and dry eye – both of which require the patient to administer eye drops consistently over a long period of time – adherence is found to be low, particularly for glaucoma, a leading cause of irreversible blindness worldwide.5-8 Nearly nine out of ten glaucoma patients are unable to instil eye drops correctly and therefore an easy-to-use system that is appreciated by patients could contribute to improving their compliance with a treatment.9 With an ageing population and an increasing number of patients suffering from chronic eye conditions, effective drug delivery and patient adherence to treatments are increasingly important considerations in ophthalmology.
Nemera’s answer to challenge the issue of poor adherence and improve the patient’s experience is the development of e-Novelia – an add-on device to Nemera’s existing preservative-free multi-dose eye dropper (Figure 4).
Using smart add-on technology, the patient benefits from digitalised and interactive instructions via their smartphone. Key features include providing patients with reminders on when to take their next dose and when to replace their medication, for example. The add-on can also track the number of drops delivered and when exactly they have been delivered (date and time), comparing the actual intake with the posology – thus calculating the patient’s compliance. This new technology will not only be useful for patients but for the entire industry. Healthcare professionals will benefit from dose-tracking analysis, researchers will be able to perform more efficient clinical studies and pharmaceutical companies will be able to launch better-performing drugs.
UNDERSTANDING THE PATIENT JOURNEY
In August 2019, Nemera acquired Insight Product Development (Chicago, IL, US) – now Insight Innovation Centre – to complement its existing innovation centre at its headquarters in La Verpillière, France.
The acquisition combines the strengths of Insight Product Development in front-end innovation, design research, human factors and design engineering with Nemera’s strong late-stage development, as well as clinical and commercial manufacturing capabilities. These newly enhanced capabilities now enable Nemera to offer end-to-end development services to all its customers globally and to collaborate with them earlier, supporting their complex drug delivery and medical device projects.
By conducting interviews in a natural home setting, familiar to the patient, Nemera is able to obtain a more accurate insight into how drug delivery devices are actually used. There is significant value to be derived from this method of understanding, characterising and prioritising user needs, which is best initially achieved through applied ethnography. This method relies on a combination of interviews and in-context observations of practices, processes and experiences within the patient’s home, clinical environment or any natural setting. This approach can yield a deep, longitudinal understanding of the patient journey – from diagnosis, to treatment selection, to onboarding and ongoing use.
A map of the patient journey provides a very powerful tool to drive inputs and decisions, especially when used in the earliest stages of a development programme. Thoroughly understanding the patient journey can then be leveraged as a critical early-stage road map to help inform selection of the appropriate device. This understanding can also help Nemera uncover patient engagement opportunities that can be supported with connectivity and mobile applications to support value-based care. Nemera firmly believes that developing a holistic and comprehensive patient experience and human factors management strategy can create significant competitive advantages and ultimately result in safe, effective and differentiated combination products that respond to patient needs.
REFERENCES
- a) Schaumberg D, Sullivan D, Buring J, Dana M, “Prevalence of dry eye syndrome among US women”. Am J Ophthalmol, 2003, Vol 136(2), pp 318–26. b) Schaumberg D, Dana R, Buring J, Sullivan D, “Prevalence of dry eye disease among US men: estimates from the Physicians’ Health Studies”. Arch Ophthalmol, 2009, Vol 127(6), pp 763–8.
- “Report of the International Dry Eye Workshop”. Ocul Surf, 2007, Vol 5(2), pp 65–204.
- Hasegawa H, Naganuma K, Nakagawa Y, Matsuyama T, “Membrane filter (pore size, 0.22- 0.45 μm; thickness, 150 μm) passingthrough activity of Pseudomonas aeruginosa and other bacterial species with indigenous infiltration ability”. FEMS Microbiology Letters 2003, Vol 223, pp 41–46.
- “User study performed for Nemera by GfK to understand the Novelia market opportunities versus competitor”. GfK report, 2015.
- Thompson A, Woolson S, Olsen M et al, “Relationship between electronically measured medication adherence and vision-related quality of life in a cohort of patients with open-angle glaucoma”. BMJ Open Ophthalmology, BMJ Open Ophthalmol, 2018, Vol 3(1), e000114.
- Welge-Lussen U, Weise S, Yu A, “Assessing the adherence behavior of glaucoma patients to topical eye drops”. Patient Preference and Adherence, 2014, Vol 18(9), pp 17-23.
- Cate H, et al, “Patterns of adherence behaviour for patients with glaucoma”. Eye (Lond), 2013, Vol 27(4), pp 545-553.
- Robin A, Grover D, “Compliance and adherence in glaucoma management”. Indian J Ophthalmol, 2011, Vol 59 (Suppl 1), pp S93-S96.
- Gupta R et al, “Evaluating eyedrop instillation technique in glaucoma patients”. J Glaucoma, 2012, Vol 21(3), pp 189–192.

