To Issue 143
Citation: Miller E, De Clercq K, “A Sight for Sore Eyes: Challenges and Innovations in Ophthalmic Drug Delivery”. ONdrugDelivery, Issue 143 (Mar 2023), pp 6–9.
Ethan Miller and Kristien De Clercq discuss recent advancements and current challenges in the field of ophthalmology, including the trend towards preservative-free multidose delivery systems, delivering smaller topical volumes with increased drug efficacy, slow-release injections for delivery to the posterior of the eye and the exciting field of gene therapy.
This article discusses some of the big challenges of ophthalmic drug delivery and provides examples of innovative solutions for more effective, targeted and sustainable treatment. With rising rates in eye-related conditions, such as dry/wet macular degeneration, conjunctivitis, glaucoma and diabetic retinopathy, global demand for ophthalmic drugs is only growing. Many ophthalmic drug delivery methods are invasive, especially when targeting areas like the vitreous humour in the posterior eye (Figure 1). Even delivery to the anterior regions, like the cornea and sclera, has its challenges with controlling dosage and preventing contamination after multiple uses. Innovations in these areas are valuable, with global sales of ophthalmic drugs at US$35 billion (£29 billion) in 20211 and expected to grow to U$48 billion by 2030.2
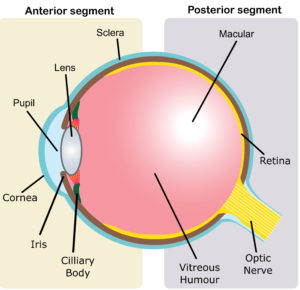
Figure 1: Schematic showing anatomy of the human eye, including the (A) anterior and (B) posterior segments.
“While there is currently a strong movement towards preservative-free eye drops and topical applications, the issue of microbial contamination must still be addressed.”
CONTAMINANT-FREE AND PRESERVATIVE-FREE
Studies have shown that 24% of eye drops that are applied by patients become contaminated with micro-organisms,3 which can cause infections that lead to significant damage. Ophthalmic indications have drawn the attention of medical practitioners for centuries, with historical evidence for the use of pastes to cure eye-related ailments.4 Since then, formulations have come a long way, but contamination remains a challenge with respect to the corneal epithelium, ulcers and even blindness.5
To deal with this, preservatives, such as benzalkonium chloride, are often added to formulations to act as antimicrobials. However, there are negative effects associated with their with long-term use. Benzalkonium chloride has been linked with Lachrymal film dysfunction, ocular hyperaemia, dotted keratitis and toxic keratopathy.6 While there is currently a strong movement towards preservative-free eye drops and topical applications, the issue of microbial contamination must still be addressed.
One way companies are overcoming this challenge is by revolutionising how eye drops are dispensed. For example, Aptar Pharma has developed a preservative-free multidose ophthalmic drug-device combination product – the Ophthalmic Squeeze Dispenser.7 This product uses a patented mechanical tipseal technology that creates a tight seal on the sterile formulation reservoir, dispensing a precise dose and filtering incoming air sterile through a 0.2 μm filter membrane. URSAPHARM (Saarbrücken, Germany) uses its COMOD system, which dispenses a single drop without introducing air or contaminants to sterile formulation, allowing for multiple safe, preservative-free doses. The ABAK® system from Théa Laboratories (Clermont-Ferrand, France) is another preservative-free multidose system, using a hydrophilic microporous pad to regulate fluid flow, dispensing single drops while filtering air and contaminants from returning solution.6
These few examples demonstrate the innovative mechanisms that are being employed to maintain formulation sterility without the use of preservatives. However, these techniques are not without their challenges – they require robust seal integrity and rigorous verification and validation to comply with regulatory requirements.7 That said, if successful, they provide preservative- and contaminant free devices that make eye care easier and more manageable for patients (Figure 2).

Figure 2: When using eye drops, precision and technique matter for effective treatment of ophthalmic conditions. Advancements in eye care are happening at an accelerating rate, especially with new technologies providing a route towards easy-to-apply, multidose devices and preservative-free formulations. (Image credit: Yaroslav Shuraev / Pexels 2021.)
THE PERFECT AMOUNT – DOSAGE CONTROL
A common struggle with the application of ophthalmic drugs is dosage. A typical eye drop is around 40 μL in volume, even though the human eye can only hold approximately 7 μL of fluid,8 which results in significant wastage and poor dosage control of therapeutics. This is further compounded by patients incorrectly dosing their drugs – a study of glaucoma patients found that 90% of patients used an improper dosing technique,9 with a significant number of them missing the eye completely.10
Kedalion Therapeutics, recently purchased by Novartis, has developed a novel multidosing method using an ideal stream. The proprietary AcuStream™ delivery system for topical ocular treatments delivers a gentle, continuous and reproducible stream of therapeutic directly to the eye, providing precision dosage.11 This means that less drug can be applied for a similar therapeutic effect, resulting in reduced drug wastage when compared with standard eyedroppers. The stream is applied horizontally, which allows patients to adopt a more natural posture, and is delivered in a fraction of a second to beat the blink reflex.
Eyenovia (NY, US) has developed a novel approach using microarray print technology in its Optejet device. This microdosing spray functions akin to an inkjet printer, generating a directional mist of microdroplets arranged to coat the corneal surface. With a typical volume of approximately 8 μL, this microdroplet array mitigates problems such as drug overflow. This method also offers increased precision, as the micro-array of droplets delivers the same amount of drug with 80% less corneal exposure, in turn reducing the risk of side-effects from preservatives.
Notably, the implementation of multidosing methods can also have positive impacts for sustainability, lowering drug and plastic waste and cutting associated carbon emissions. A recent study showed that consumer plastic waste can be reduced up to 7.5 times by switching from single- to multidose methods, helping tackle the issue of blow-fill-seals going to landfill waste.12 These novel multidose delivery systems are just a few examples of how such technologies can minimise incorrect dosing, simplify patient care and enhance the sustainability of the healthcare system. “While there is currently a strong movement towards preservative-free eye drops and topical applications, the issue of microbial contamination must still be addressed.”
“Intravitreal injections minimise systemic exposure but are not without risks, requiring careful patient monitoring and precise injection technique to minimise the potential for complications.”
SLOWER DRUGS, FEWER INJECTIONS
Another way in which novel drug delivery systems are revolutionising the field of ophthalmology is by providing clinicians with more effective treatment options for ocular diseases. Intravitreal injections are injections are administered directly into vitreous humour (Figure 1) and are a common method for treating a range of diseases within the posterior of the eye, such as wet age-related macular degeneration. Intravitreal injections minimise systemic exposure but are not without risks, requiring careful patient monitoring and precise injection technique to minimise the potential for complications. These risks are only compounded when multiple injections are required for a single course of treatment.
The use of slow-release drugs is especially beneficial for posterior eye treatments as they enable sustained and controlled drug release over a long period of time, leading to better treatment outcomes, reduced dosing frequency and minimised side effects. For example, Ozurdex from AbbVie is a biodegradable intravitreal implant designed for the treatment of macular oedema and uveitis. The implant slowly releases dexamethasone, a potent corticosteroid, over a period of several months, providing sustained therapeutic effects. A single intravitreal injection is required to position the implant, significantly reducing the frequency of injections, and the device dissolves naturally so there is no need for additional surgery to remove the implant when treatment is finished.
VisusNano (London, UK), which was recently awarded £1.5 million in funding, has developed a drug-eluting artificial intraocular lens implant – MEDILens – for patients undergoing cataract surgery. The novel biodegradable polymer-based lens enables the slow, controlled release of antibiotic, anti-inflammatory and anti-proliferative agents, improving patient convenience.
Even purely mechanical solutions can be implemented, such as the iStent manufactured by Glaukos (CA, US). The iStent reduces intraocular pressure in patients suffering from open-angle glaucoma via a biocompatible titanium implant that improves the outflow of aqueous humour from the eye, thereby reducing pressure on the optic nerve.13
Innovative technologies for the delivery of drugs to the posterior region of the eye continue to be developed, creating new opportunities to provide better care and treatment to patients with ocular disorders, while also reducing associated risks, complications and providing more options to clinicians.
“Gene therapy could provide a means to prevent, cure and even reverse many ocular disorders, using the body’s own cellular machinery to restore vision impairments untreatable by conventional means.”
THE FUTURE IS BRIGHT WITH GENE THERAPY
Upcoming innovations in ophthalmic drug delivery are likely to include gene therapy, an approach that involves the delivery of functional genes to affected cells in the eye. Genes are the instructions for how living things develop and function, providing the blueprints on how to make the specific proteins that are required for various functions of the body. Gene therapy acts to replace or repair defective genes – in the case of ophthalmology, restoring visual function.
Research on new methods to deliver the genes to cells is ongoing, such as by using the natural ability of viruses to penetrate and hijack cells, and using them to deliver therapeutic genes instead of their own genetic material (Figure 3). A serum containing such viruses, or “vectors”, would typically be injected into the target tissue to initiate treatment.

Figure 3: Gene therapy is a rapidly developing field of research. Using vectors, genetic material is delivered to the affected cells in the eye, using their own internal machinery to counteract targeted ocular pathologies. (Image credit: Edward Jenner / Pexels 2020.)
However, many challenges remain in this field of development. For example, to achieve the desired results, therapies must contain a complex cocktail of agents, including tissue-specific promoters, enhancers and other factors involved in targeted transcription and translation.14 Advances are being made, with new vectors and gene delivery systems making good progress – for example, inhibitors of the RTP801 gene for treating microvascular illness16 and a treatment for Leber congenital amaurosis, a severe form of inherited blindness, using adeno-associated virus vectors to deliver a functional copy of the RPE65 gene to retinal cells.15
Ongoing clinical trials are providing encouraging results and suggest that this approach could indeed revolutionise how ocular disorders are treated in the near future. Gene therapy could provide a means to prevent, cure and even reverse many ocular disorders, using the body’s own cellular machinery to restore vision impairments untreatable by conventional means.
CONCLUSION
In conclusion, the field of ophthalmic drug delivery has seen significant advancements in recent years, with new technologies and innovations addressing longstanding industry challenges. Preservative-free multidose systems have made it easier to administer drugs to patients, while simultaneously reducing the risk of adverse reactions and improving patient comfort; the application of sustained- and controlled-release drugs means many treatments to the posterior region of the eye now require fewer intravitreal injections while remaining just as effective; and gene therapy is showing promising results in treating inherited eye diseases. These advancements are greatly benefiting patients and providing more effective treatments for a range of eye conditions.
Although challenges related to personalised treatments, bioavailability and sustainability still need to be addressed, it can be anticipated that the emergence of further innovative solutions will enhance the safety and efficacy of ophthalmic drug delivery.
REFERENCES
- “Ophthalmology Drugs Market accelerating at a CAGR of 7.2% from 2023 to 2030”. Pharmiweb, Jan 2023.
- “Ophthalmic Drugs Market Report to 2030”. Visiongain, Nov 2020.
- Daehn T et al, “Contamination of multi dose eyedrops in the intra and perioperative context”. Sci Rep, 2021, Vol 11, article 20364.
- Abelson MB, Lafond A, “3,500 Years of Artificial Tears”. Review of Ophthalmology, Dec 2014.
- da Costa AX et al, “Microbial Cross-contamination in Multidose Eyedrops: The Impact of Instillation Angle and Bottle Geometry”. Transl Vis Sci Technol, 2020, Vol 9(7), p 7.
- Coroi MC, Bungau S, Tit M, “Preservatives from the Eye Drops and the Ocular Surface”. Rom J Ophthalmol, 2015, Vol 59(1), pp 2–5.
- Ritter A, Birkhoff M, “An Eye on the Future of Preservative-Free Drops”. ONdrugDelivery, Issue 130 (Mar 2022), pp 20–26.
- Hardy A, “Eyenovia micro-array print therapeutics targeting various ophthalmic indications”. BioTuesdays, May 2020.
- Stone JL et al, “An objective evaluation of eyedrop instillation in patients with glaucoma”. Arch Ophthalmol, 2009, Vol 127(6), pp 732–736.
- Rotchford AP, Murphy KM, “Compliance with timolol treatment in glaucoma”. Eye (Lond), 1998, Vol 12(Pt 2), pp 234–236.
- Noymer P et al, “Acustream™: Bringing Topical Ophthalmic Drug Delivery into the Modern Era”. ONdrugDelivery, Issue 94 (Jan 2019), pp 32–35.
- Govindasamy G et al, “Limiting plastic waste in dry eye practice for environmental sustainability”. Ocul Surg, 2022, Vol 25, pp 87–88.
- Samuelson TW, “iStent Trabecular Micro-Bypass Surgery”. American Academy of Ophthalmology, Nov 2017.
- Souto EB et al, “Advanced Formulation Approaches for Ocular Drug Delivery: State-Of-The-Art and Recent Patents”. Pharmaceutics, 2019, Vol 11(9), p 460.
- Bainbridge JWB et al, “Effect of gene therapy on visual function in Leber’s congenital amaurosis”. N Engl J Med, 2008, Vol 358(21), pp 2231–2239.

