To Issue 181
Citation: Sanchez A, Dean C, “Drug Delivery Device Innovation in Cell and Gene Therapies”. ONdrugDelivery, Issue 181 (Dec 2025), pp 12–14.
Dr Alejandra Sanchez and Charlie Dean consider the evolution of drug delivery technologies and how cell and gene therapies are showing promise as a new class of medicine to correct disease at its source.
Drug delivery devices do more than deliver medicine into the body – they enable breakthroughs in treatment. Their evolution has always been closely tied to the therapies they support and the diseases they treat. As medicine has moved from pills to complex biologics, and now to cutting-edge cell and gene therapies (CGTs), the ways that these treatments are delivered have had to keep up. For pharmaceutical companies, device developers and innovators in the drug delivery space, the advent of CGTs presents a turning point full of exciting challenges.
EVOLUTION OF DRUG DELIVERY TECHNOLOGIES
For decades, small molecules dominated medicine – stable compounds primarily administered as pills and easily absorbed through cell membranes. When biologics arrived, they rewrote the rules. These large, delicate molecules cannot survive the gut, which makes oral delivery mostly impossible and injections essential.
“THE ONGOING BIOLOGICS BOOM OVER THE PAST DECADES SHOWS WHAT EFFECTIVE COLLABORATION AND INNOVATION CAN ACHIEVE.”
The ongoing biologics boom over the past decades has shown what effective collaboration and innovation can achieve. The need to improve adherence and the patient experience drove the development of autoinjectors, pen devices and connected drug delivery systems. Features such as needle safety, variable dosing and temperature monitoring transformed treatment, making it safer, more reliable and more convenient. These advances did not just improve care; they enabled new commercial models and patient-engagement strategies that reshaped entire markets.
Now, as the industry enters the era of CGTs, the stakes are even higher. The need for user-centric innovation remains, but the complexity, cost and risk demand solutions that go far beyond what has worked before.
THE CHALLENGE OF LIVING MEDICINES
CGTs represent a new class of medicine – living or genetic treatments that repair, replace or reprogram cells to correct disease at its source. Unlike traditional drugs, these therapies are not mass-produced or repeatedly dosed. They are usually tailored to individual patients and delivered as one-time, potentially curative interventions, often described as “one-and-done” solutions.
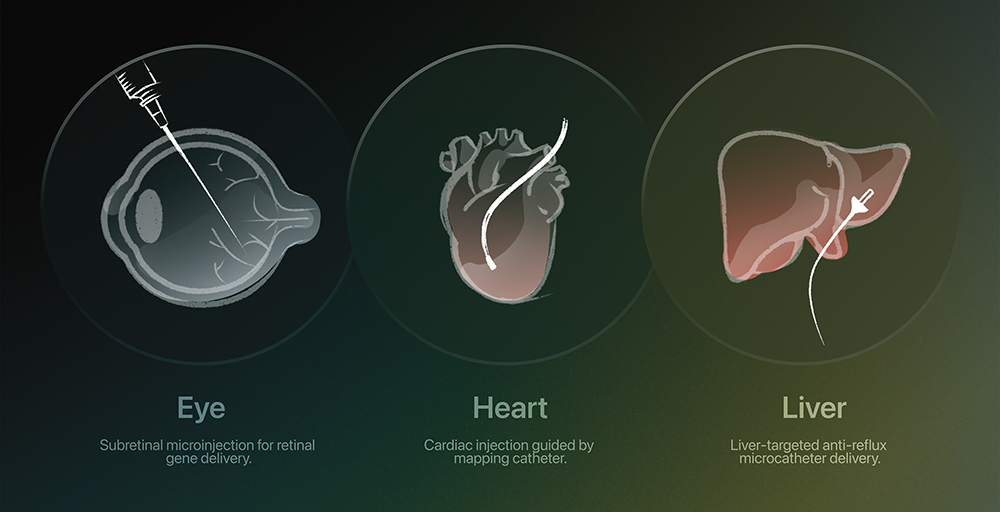
Figure 1: Precision routes for CGTs – tailored delivery systems are designed to accurately target specific organs and tissues.
Delivering such treatments, however, is far from simple. CGTs are fragile, some of them requiring cryogenic storage and careful handling to preserve viability. Even administration poses unique hurdles – most must be infused by specialists in controlled environments, sometimes directly into target tissues such as the retina, liver or heart (Figures 1 & 2).
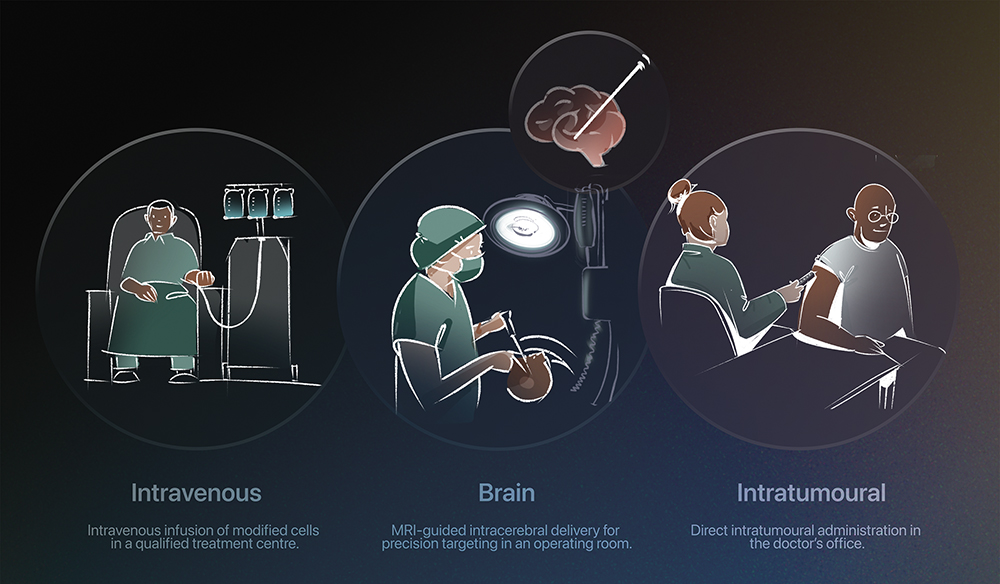
Figure 2: Each therapy requires a specialised clinical setting for safe and effective delivery.
“PRECISION AND PROTECTION ARE EVERYTHING. WHILE MANY EXISTING CGTS ARE DELIVERED THROUGH INTRAVENOUS INFUSION, OTHERS REQUIRE HIGHLY SPECIALISED DEVICES TO ENSURE ACCURACY AND CELL VIABILITY.”
Precision and protection are everything. While many existing CGTs are delivered via intravenous infusion, others require highly specialised devices to ensure accuracy and cell viability. For instance, chimeric antigen receptor t-cell (CAR-T) therapies, such as KYMRIAH (tisagenlecleucel, Novartis) and Yescarta (axicabtagene ciloleucel – Kite Pharma, Santa Monica, CA, US), are administered as one-time intravenous infusions within certified treatment centres under strict handling protocols. In contrast, LUXTURNA (voretigene neparvovec-rzyl – Spark Therapeutics, Philadelphia, PA, US) is delivered under anaesthesia in a controlled surgical environment using a customised subretinal injection tool and advanced vitreoretinal techniques. Similarly, KEBILIDI (eladocagene exuparvovec-tneq – PTC Therapeutics, Warren, NJ, US) requires precise intraputaminal administration, involving the creation of a small entry point through the skull and dura to safely reach the target brain region.
Technology is pushing the boundaries even further. Mapping catheters are now being explored to guide cardiac injections with millimetre accuracy, while hepatic-artery and intracoronary microcatheters enable precise gene delivery to the liver and heart. Anti-reflux microcatheters, which temporarily block blood flow to prevent vector leakage, are also improving targeting efficiency.
Most currently approved CGTs focus on rare diseases, but the next wave aims to tackle more common conditions such as Parkinson’s disease and dementia. In the UK alone, the number of patients eligible for these treatments could rise from 2,500 in 2021 to around 10,000 per year by 2028.1 As these therapies scale, delivery will need to become safer, more efficient and more accessible.
FUTURE OUTLOOK: BEYOND SPECIALISED CLINICS
Inspired by delivery systems for small molecules and biologics, advanced therapies are now edging closer to patients with greater safety and precision (Figure 3). Emerging platforms – from microneedle patches delivering complex biologics, such as RNA payloads, to wearable injectors for large-volume infusions and inhaled gene therapy systems – are beginning to redefine what is possible. Bedside delivery systems are also being developed to enable both in vivo and ex vivo treatments directly at the point of care. While many of these technologies remain in early stages, they signal a clear shift towards decentralised care, where device engineering, formulation science and digital health converge to make CGTs more scalable, accessible and patient centric.
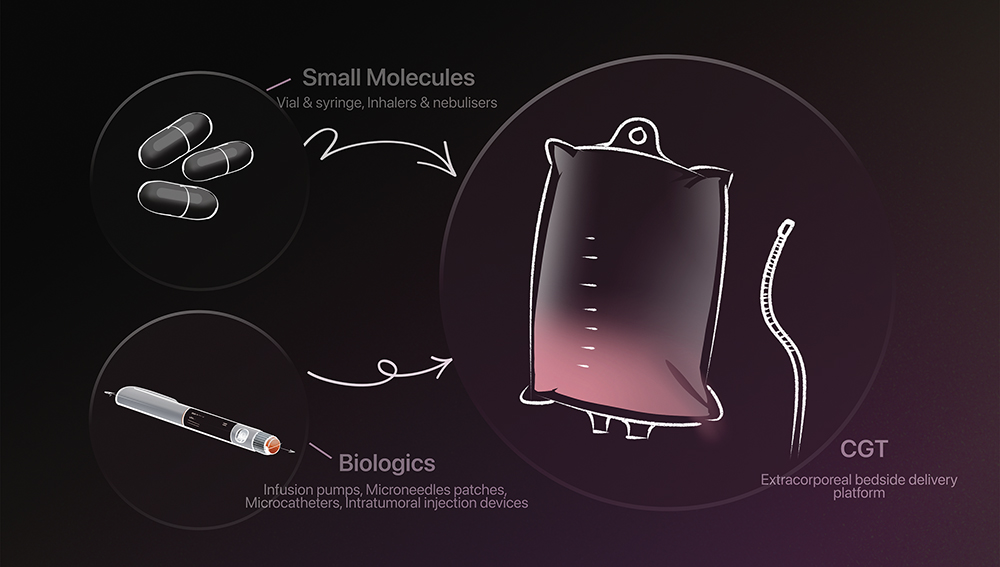
Figure 3: Examples of systems once built for more conventional therapies that are now being reimagined for cell and gene treatments, with new innovations still unfolding as these therapies advance.
The Next Chapter in CGT
While the experience gained from biologic drug delivery offers valuable insights for CGTs, these advanced treatments require a fundamentally different approach. With biologics, treatments are typically used for chronic conditions, where patient convenience, usability and long-term adherence are key to therapeutic success. In contrast, CGTs are often one-time, highly personalised interventions, where the priority shifts to safeguarding the viability, sterility and potency of living cells or viral vectors.
Unlike conventional medicines, most CGTs are “one-and-done” therapies, where a single dose can correct a genetic fault for life. This changes everything. Success is no longer about adherence or cost efficiency but about protecting the therapy’s integrity from the moment it leaves the manufacturing line (or the patient or donor’s body) until it reaches its destination. Every step – storage, transport, thawing and infusion – must safeguard the therapy’s potency and stability.
To meet this challenge, next-generation devices are being engineered to protect delicate materials from mechanical and thermal stress by using low-shear pumping, temperature-controlled flow paths, and integration with cryogenic storage and thawing systems. Closed, single-use designs now minimise contamination risk, while embedded sensors and connected data platforms enable real-time tracking and control throughout the therapy’s journey.
Looking ahead, innovation will hinge on simplifying complexity while maintaining viability. Redesigning delivery routes, whether for the brain, retina or heart, requires sub-millimetre precision and image-guided accuracy, yet future progress may come from rethinking administration itself – exploring minimally invasive or alternative routes, closed-loop systems and intelligent feedback mechanisms that could one day make bedside, or even at-home, CGT delivery possible.
Ultimately, the further evolution of delivery technology will determine how far these therapies can go. For CGT, the device is no longer just a vessel, it is the guardian of the cure and a critical enabler of therapeutic success.
REFERENCE
- “Unlocking access to future ATMPs in the UK: Comparing international approaches”. ABPI, Jul 2024.

