To Issue 177
Citation: Pearce G, “DuoVIAL® – Protect, Mix and Deliver”. ONdrugDelivery, Issue 177 (Sep/Oct 2025), pp 44–49.
Gareth Pearce introduces Pacifi‘s DuoVIAL® primary packaging technology for topical lyophilised formulations – a minimalist dual-chamber drug delivery system that enables safer, easier and more cost-effective reconstitution, while retaining the proven materials, formats and filling processes well established in the pharmaceutical sector.
With a growing pipeline of biologics, lyophilised formulations can provide the necessary stability to help support drug efficacy. It is, however, worth recognising that it is not only parenteral drug products that benefit from lyophilisation. Within topical therapies, there are now live biological products (LBPs) for treating inflammatory skin conditions undergoing clinical trials. Notably, the end user is not a skilled healthcare professional, and meeting the challenges of reconstitution in a non-clinical environment requires serious consideration of the packaging format and delivery mechanism.
The global lyophilised drugs market is estimated to be valued at US$371 billion (£275 billion) in 2025 and is expected to reach $683 billion by 2032, exhibiting a compound annual growth rate of 9.1% from 2025 to 2032. By packaging type, it is expected that the vial segment will continue to lead the lyophilised drugs market in 2025, with a projected contribution of 40.5%.1 While the majority of lyophilised drug products will be processed in traditional tubular glass vials, dual-chamber syringe and cartridge formats are anticipated to see continued growth, due to increased end-user convenience. While bulk lyophilisation in trays, milling and transfer of powder into specialist packaging is established, a more exciting lyobead technology appears to be able to achieve increased productivity, reduced costs, lower cycle times and easier handling.2
RISK MANAGEMENT
The technologies underpinning Pacifi’s innovative approach were conceived in response to an ampoule sharps injury complaint, frustration by market acceptance of the status quo and acknowledgement of the inertia of a conservative pharmaceutical industry. In cases where ampoules and vials are the assumed packaging of choice for ease of use and safety, there are a number of accessories that are usually recommended to supplement them. However, human nature and convenience often mean these accessories are treated as optional; for example, using a blunt filter needle to draw product from an ampoule to mitigate the risk of glass particles and needlestick injuries, or with a vial to mitigate particles from coring the rubber stopper entering the drug product.
“IT OFTEN FEELS LIKE THESE ADDITIONAL COMPONENTS, COMPLEXITIES AND COSTS ARE A STICKING PLASTER THAT DO NOT ADDRESS THE FUNDAMENTAL FLAWS IN TRADITIONAL PRIMARY PACKAGING FORMATS.”
Vial-to-vial reconstitution can benefit from the use of sophisticated vial adaptors that incorporate double particle filters, visual cues, and functional and ergonomic features that can help to mitigate sharps injuries, particles, user errors and non-aseptic technique. However, it often feels like these additional components, complexities and costs are a sticking plaster that do not address the fundamental flaws in traditional primary packaging formats. Alternatively, there are proprietary dual-chamber syringe and cartridge solutions that may be easier and safer, but are typically only commercially viable for premium drug products.
Meanwhile, regulatory hurdles continue to increase; the US Pharmacopoeia has evolved to enable a more considered approach to drug delivery device innovation based upon assessing risk and resolving it through suitable design solutions and systems. Notably, in the past, glass flakes arising from delamination, driven by the incompatibility of specific formulations, process conditions and glass material, led to studies and proposals to use vial adaptors incorporating filters.3 Not every administration route has the acceptance quality limits specified for sterile injectables – topical and oral therapies are aligned with fitness for purpose when it comes to inspection and particles.

Figure 1: Ampoule + Vial = DuoVIAL®.
FORM FACTOR
Is it a vial? Is it an ampoule? No, it’s DuoVIAL® (Figure 1). Combining the functional advantages of ampoule and vial into a dual-chamber system, enhanced by laser technology, Pacifi’s innovation brings DuoVIAL® to market, a unique, patented primary packaging system.4
The benefits are that the primary materials, manufacturing processes, form factors, filling and sealing remain broadly similar to those for vials and ampoules, with the exception of introducing a unique laser process – Lasered Annular Cleave Ring (LACR™) – to modify and pre-weaken the glass membrane in a much more precise and controlled way than traditional scoring. The form is also relatively scalable, in terms of diameter, length and chamber ratio to accommodate unit- and multidose and mix-ratio variations.
Simplistically, a single-piece tubular glass form incorporates a LACR’d glass membrane separating the two chambers. The openings at each end can be formed with their respective finish, be it a flange (stopper and seal), thread (screw cap), cylinder (piston) or flared ampoule opening (flame sealed). The vial end, having the smaller inner chamber, may contain the lyophilisate and be extended for increased fill volume, while maintaining a standard neck finish diameter. This minimalist form, produced on relatively conventional glass-forming lines is cost effective, while also delivering an advantageous new format for lyophilised formulations.
The ampoule end may have a thin-wall flared finish, typical of ampoules, enabling a hermetic glass seal through flame sealing. Advantageously, the diluent is only ever in contact with the relatively inert glass during storage. No butyl rubber or siliconisation is required, mitigating the formulation compatibility challenges arising from leachables or silicone oil. This patented packaging system is equally applicable to both glass and polymers, enabling a broader choice of materials, whether driven by barrier performance, formulation compatibility, cost, situational usage, functional features or sustainability drivers.
LACR & SIFT – ENABLING TECHNOLOGIES
During manufacture, specialist LACR technology is applied to the glass membrane to enable the clean-cleave detachment of a contact lens-like disc, wherein any intrinsic sub-visible particles are managed through the Sintered Integral Filter Technology (SIFT™). Notably, this means that all products dispensed from DuoVIAL® are filtered before administration.
LACR is a unique process applied through a clean, non-contact, high-speed, focused beam of light modifying the material. This confidential patented process is light years ahead of traditional glass pre-weakening, ampoule scoring, one point cut, colour break ring or ablation processes. As a highly scalable process, it is both efficient and cost effective in volume production. Having applied and proven the LACR process with Type 1 borosilicate glass samples, further studies are to be conducted with the DuoVIAL® technology. In particular, the aim is to assess transparent polymers, where the injection and associated blow-moulding processes can enable enhanced features, an extended range of form factors and a broader range of applications.
In surgical adhesive and wound care applications, there are medical devices that incorporate glass “onion skin” ampoules to protect liquid formulations in storage with the glass capsule crushed to release the liquid immediately prior to use. In these devices, a filter to ensure that the sterile liquid being applied is fit for purpose and compliant with regulations is integral to the device flow path. Pacifi’s SIFT approach is very similar, with value-added design in the form of special SIFT.2K™ applicator tips, incorporating filtration, aesthetic and ergonomic form to apply product to the skin, along with venting to enable flow from the container.

Figure 2: LACR and SIFT technologies – cleavage and filtration.
Figure 2 shows a DuoVIAL® 2.5 mL cartridge incorporating a SIFT tip protruding through the glass membrane, having opened a pathway between the powder and liquid chambers, through transfer of force to cleave the disc. The SIFT.2K applicator tip emphasises the aqueous liquid (blue) wicking to the domed surface to be applied to the skin, in contrast with the vent (white) mitigating vacuum and enhancing flow. Having been activated, the clean-cleaved membrane hole has an aesthetic “frosted” surface, a positive consequence of the LACR process. The X-Ray computed tomography image of the cleaved disc emphasises the results of this unique precision process, where the cleaved frosted surface (green, Ra < 1µm), combined with the rounded rim, presents a smooth form, similar to weathered pieces of glass washed up on a beach.
“THE PRIMARY OBJECTIVE OF DUOVIAL® IS TO MAINTAIN THE STABILITY OF THE LYOPHILISED DRUG PRODUCT FROM MOISTURE DEGRADATION. GLASS, BEING UNIQUELY IMPERMEABLE AND TRANSPARENT, REMAINS THE IDEAL MATERIAL FOR THIS PURPOSE; ALTERNATIVELY, TRANSPARENT POLYMERS MAY ALSO BE USED.”
PROTECT
The primary objective of DuoVIAL® is to maintain the stability of the lyophilised drug product from moisture degradation. Glass, being uniquely impermeable and transparent, remains the ideal material for this purpose; alternatively, transparent polymers may also be used. With a first application in topical probiotic therapy, consisting of a lyophilised LBP and aqueous diluent prebiotic, the DuoDERM™ system was conceived as a single-use glass cartridge. The ampoule end is hermetically flame-sealed similar to standard ampoules and the vial end is sealed with aluminium foil by induction.
Accelerated stability studies were conducted on a DuoDERM 2.5 mL cartridge containing bacteria preserved in lyophilised powder form over an eight-week period at 40°C and 75% relative humidity, equivalent to two years of real-world conditions (Figure 3). The headspace was filled with nitrogen to displace the atmospheric air. Samples were analysed, confirming a moisture level stabilising at <3% water by weight. Subsequently the live bacteria were reconstituted and analysed to determine the cell survival rates, which remained consistently high.
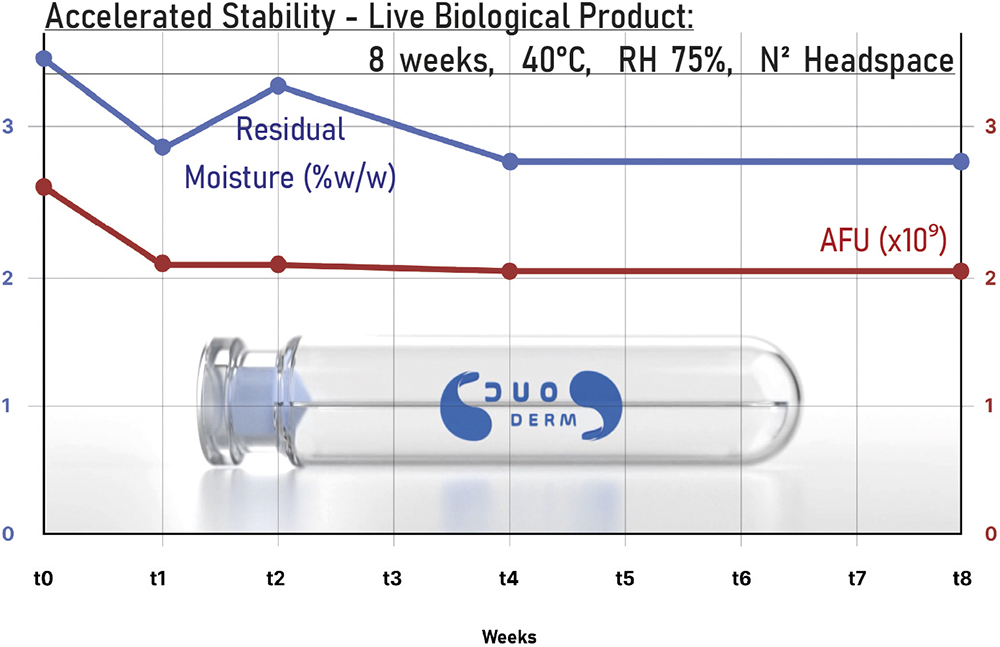
Figure 3: PROTECT – accelerated stability in a DuoDERM cartridge.
Protecting the patient, healthcare provider and planet are also potential benefits achieved through DuoVIAL®. The technology ensures administration of safe drug product, mitigates sharps injuries and reduces packaging logistics, material use, waste and costs.
MIX
DuoVIAL®’s integral glass membrane is 100% impermeable, keeping the lyophilisate dry and the diluent wet. While there are some attractive dual-chamber systems on the market where frangible polymer membranes are used, some of the common challenges they face include:
- A deliberately thinner wall section, which undermines the intended moisture barrier function
- All polymers are permeable to a greater or lesser degree.

Figure 4: MIX – lyobeads and diluent combined.
These need to be taken in context – depending upon a formulation’s sensitivity to moisture degradation, specific polymers may be fit for purpose. One solution to these challenges is to incorporate a desiccant in the lyo-chamber; however, this approach has both a finite moisture capacity and also draws moisture from the liquid chamber, increasing the liquid formulations’ viscosity, lessening its ability to mix and pour. This complex matrix of materials also undermines recyclability initiatives.
For DuoDERM, The LACR’d membrane being pre-weakened allows the disc to be displaced, opening a porthole in the membrane, which allows the liquid and lyophilisate to combine and reconstitute. Agitating the container enhances the mixing process. Figure 4 shows a DuoDERM 2.5 mL cartridge with incorporated lyobeads, alongside an aqueous diluent, separated by the transparent membrane. Aluminium foil is induction-sealed to complete the impermeable lyo-chamber, while the trio of 2.5 mL cartridges contain a lyophilised milled powder and the aluminium foil seal secures the lyophilisate, with the diluent hermetically sealed by flame.
“TO MANAGE THIS AND PROVIDE DISPENSING FLEXIBILITY,
DUODERM CAN INCORPORATE DIFFERENT APPLICATOR TIPS AND DISPENSING MECHANISMS.”
DELIVER
Each formulation is tailored to its administration route and therapy, presenting individual challenges in terms of dosing, whether it be unit-dose or multidose; aqueous-, alcohol- or oil-based; or a solution, emulsion or suspension. In all cases, a key consideration is viscosity. To manage this and provide dispensing flexibility, DuoDERM can incorporate different applicator tips and dispensing mechanisms. In its simplest form, the base is a flame-sealed dome, whilst opposite is a SIFT.2K domed form that filters and wicks the solution to be applied directly to the skin. Alternatively, an airless pump format may be desirable to help protect the formulation from environmental contamination and degradation once activated and during the period of use. Another option is a syringe format with graduations, where the end-user depresses the plunger rod to dispense product via a cannula-like nozzle.
Figure 5 shows the DuoDERM system in its simplest cartridge form, incorporating the DuoVIAL® packaging technology, including a reusable applicator for activation and administration of therapies contained in single-use cartridges. The SIFT.2K tip transfers user-applied cap torque, cleaving the glass membrane, enabling reconstitution, while wicking the solution to the tip where it is applied to the skin.
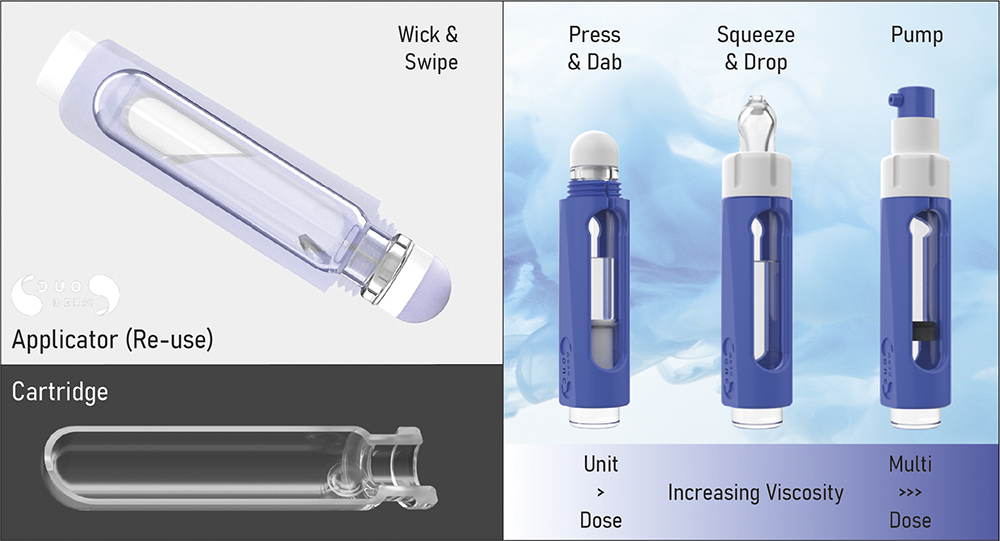
Figure 5: DELIVER – topical applicator portfolio.
Although DuoVIAL® offers benefits across sectors from diagnostics to cosmetics, its primary focus is healthcare, with broad therapeutic and administration potential. The long-term goal is parenteral vaccines; however, the initial focus is topical therapies. In dermatology, conditions such as atopic dermatitis, eczema and acne affect large populations, where conventional small-molecule treatments, such as steroids, carry undesirable side effects.
An emerging alternative – restoring skin microbiome balance via topical applications of commensal live bacteria are advancing through clinical trials. Like vaccines, these LBPs benefit from lyophilisation for stability. In this case, an unskilled consumer is required to reconstitute their medication in a non-clinical environment and apply as prescribed. However, existing pharmaceutical packaging is often unsuitable or carries unacceptable consumer risk. DuoDERM, incorporating DuoVIAL® technology, addresses these challenges, providing a safe and easy-to-use packaging system.
REFERENCES
- “Lyophilized Product Market Analysis & Forecast 2032”. Market Report, Coherent Market Insights, Feb 2022.
- “Investment in Lyobeads – A Massive Step Forward for Lyo R&D”. Web Page, Biopharma Group, accessed Aug 2025.
- Zarour-Shalev EH et al, “Filtration of Glass Delamination Particles with West Pharmaceutical Vial Adapters”. PDA J Pharm Sci Technol, 2015, Vol 69(6), pp 669–676.
- Pearce G, “A method of packaging a two component composition into a dual vial”. Patent GB2569984A, Pacifi Ltd.

