To Issue 133
Citation: Neely M, “Enabling a New Paradigm for Peptide Drug Development”. ONdrugDelivery, Issue 133 (May 2022), pp 22–25.
Michael Neely reviews peptide formulations and introduces Xeris’s XeriSol™ technology and its potential paradigm-changing capabilities.
Peptides now comprise some of the most important therapeutics in the modern pharmacopoeia. The advent of simplified and economical synthesis routes, tools for rapid laboratory synthesis, and advances in large-scale production technologies for synthetic peptides have enabled an extensive discovery and development effort across the pharmaceutical industry. Peptide drugs now account for an estimated US$28.5 billion (£21.8 billion) of worldwide pharmaceutical revenues and include hundreds of products, ranging from orphan drugs to drugs used by millions of patients on a daily basis. Growth is expected to continue at 9.6% over the next several years.1
Peptide molecules in traditional aqueous formulations are subject to various physical interactions that present formulation challenges. For example, they will undergo hydrolysis rather quickly in the presence of water and, in useful concentrations, will often precipitate or form aggregates that diminish or destroy their pharmacological activity while also raising immunogenicity concerns. Depending on pH requirements, peptides as aqueous solutions for injection may reach a point where they become painful due to high acid levels.
Early peptide formulations, including many that are still marketed, relied on lyophilisation of the peptide to enable packaging in vials and sufficient stability to allow distribution through the supply chain. Lyophilisation is a capital-intensive unit operation that demands both a high level of energy consumption and significant developmental effort to achieve the required process parameters. Furthermore, lyophilised products require reconstitution with a sterile diluent at the point of use, adding complexity and introducing the opportunity for dosing errors or contamination to occur.
Because peptides are labile to acids, the bases and proteases that are present in the alimentary canal mean that oral administration is not directly feasible. The simplest and most viable routes of administration have been, and will remain, parenteral; either subcutaneous, intramuscular or intravenous. Industry and academia have collectively invested billions of dollars over the past decade and a half chasing the “holy grail” of oral peptide delivery. There have been some significant successes, but they all come at a cost.
Typical oral formulations of peptides rely on a significant excess of the active ingredient to deliver a required dose across the gut, through the liver and into systemic circulation. Chemical modifications have been developed that help the peptide survive this gauntlet, as well as microencapsulation technologies towards the same end. Each of these approaches adds cost and complexity to clinical development, manufacturing processes and the overall cost to achieve approval and, ultimately, reach markets. In competitive markets, the cost of this kind of product differentiation effort may or may not be adequately rewarded. Much depends on the willingness of third-party payers to agree that the benefits of novel formulations merit the cost. A similar situation occurs for other routes of administration, such as pulmonary or transdermal delivery. Getting an accurate dose into the target tissues requires significant time, money and effort, as well as no small measure of good fortune.
“Science has given the pharmaceutical industry a vast and growing library of pharmacologically useful peptides, so one might ask if there is a different developmental paradigm for these potentially vital medications that might serve the cause of medicine better?”
Science has given the pharmaceutical industry a vast and growing library of pharmacologically useful peptides, so one might ask if there is a different developmental paradigm for these potentially vital medications that might serve the cause of medicine better? Given that, for any therapeutic biomolecule, parenteral administration is virtually always simple and efficacious, would not a more perfect method of producing parenteral doses be of great value? The term “elegance” is used in mathematics to describe the simplest and most straightforward way of constructing a proof of a theorem. There is, in fact, a more elegant solution available to those who propose to formulate and market peptide drugs. That solution is Xerisol™, a peptide and small molecule drug formulation platform developed and made available to license through Xeris Pharmaceuticals. For those in the pharmaceutical industry focused on delivering maximum patient benefits quickly, efficiently and cost effectively, XeriSol’s properties make a compelling case.
The essence of a Xerisol formulation is comprised of the active peptide molecule and appropriate molar ratios of compatible ionic species, sometimes including an excipient, all dissolved in a polar, aprotic (non-aqueous) solvent. In this context, the peptide is as free from all of the deleterious effects of water as it would be in a lyophilised state, yet, being in liquid form, it remains amenable to injection. In addition to glass, the solution has been demonstrated to be compatible with many of the alternative materials commonly used to produce vials, syringes, autoinjectors, cartridges and other primary drug containers. Virtually any of the new devices that have been developed to simplify the injection process and make it more patient friendly can be compatible with a XeriSol formulation. Clinical- and commercial-scale manufacturing is similar to that of aqueous solutions and available through multiple contract development and manufacturing company organisations. XeriSol is also supported and protected by an extensive patent estate, providing an opportunity for drug developers to achieve or maintain market exclusivity for a drug product.
“Another benefit of Xerisol formulations is that they allow higher drug concentrations and, therefore, enable smaller injection volumes for a given dose of drug.”
The efforts to develop non-parenteral routes of administration for peptide drugs is driven by the supposition that injection delivery is the least favourable for patient acceptance and compliant use. That may be, but as injection technologies become more refined,that perceived barrier almost certainly becomes lower. If a few seconds of touching a device to one’s abdomen or thigh, with no perceptible pain, achieves effective delivery of a drug that provides appreciable benefits, that barrier may be less significant than currently supposed. Another benefit of Xerisol formulations is that they allow higher drug concentrations and, therefore, enable smaller injection volumes for a given dose of drug. A common complaint of injection site irritation has been demonstrated to be no more frequent with XeriSol formulations than with aqueous formulations of the same drug, and the absence of pH and reduced injected volume afforded by Xerisol might be expected to lessen such occurrences.
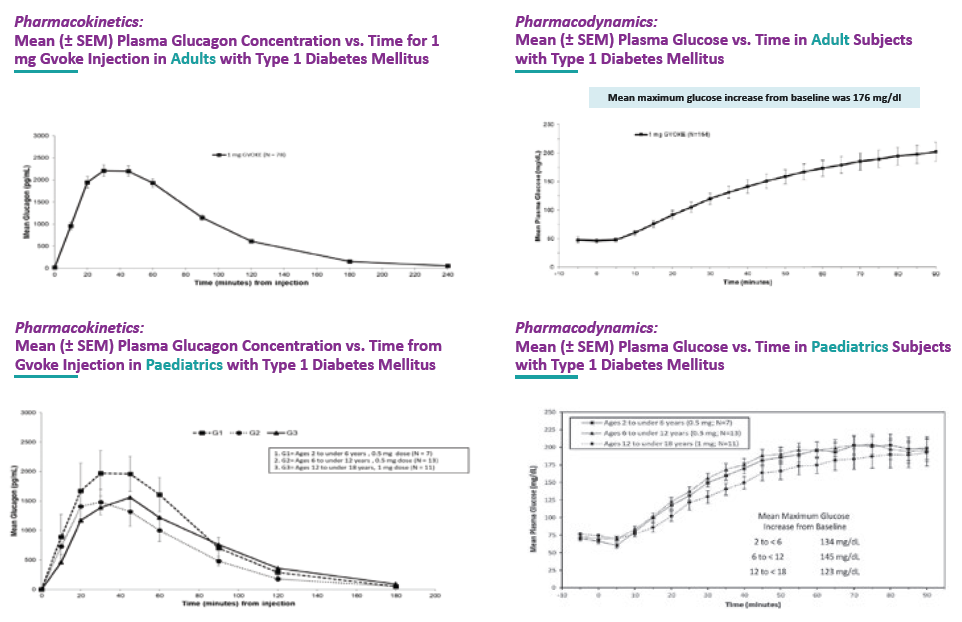
Figure 1: Pharmacodynamics of a Xerisol formulation of glucagon.
Figure 2: Gvoke glucagon in a Xerisol formulation pen injector, approved in 2020 for marketing in the US.
“Molecules that require differing pH for solution in water can be solubilised together in a Xerisol formulation because pH is obviated in the aprotic environment.”
Not only does Xerisol afford a lower cost and faster route to an effective and marketable drug product but it is also a lower risk option. Xerisol is now a clinically proven and globally approved technology. Xeris Pharmaceuticals has launched Gvoke® and Ogluo® glucagon injections in the US and European markets, respectively, for the treatment of severe hypoglycaemia in adults and paediatric populations down to the age of two (Figure 1). The excellent stability of Gvoke and Ogluo has enabled the development of prefilled syringe and autoinjector presentations (Figures 2 & 3). Xeris Pharmaceuticals is also developing XeriSol formulations to treat additional hypoglycaemic indications that require chronic exposure and has thus developed an extensive safety database. The company has conducted several nonclinical repeat-dose toxicity studies with XeriSol, including 26 weeks’ subcutaneous dosing in rats and 28 weeks’ subcutaneous dosing in minipigs. No systemic or organ toxicity was observed in animals treated with XeriSol.
Figure 3: The Gvoke glucagon prefilled syringe product
received FDA approval in 2019.
The ability to co-formulate two peptides with differing solubility characteristics in a single stable liquid presentation at high concentrations and with no adverse impact on clinical efficacy is another key enabling feature of Xerisol that would enable the development of single-dose peptide “cocktail” drugs for vaccines, hormonal control medicines or anticancer drugs. Molecules that require differing pH for solution in water can be solubilised together in a Xerisol formulation because pH is obviated in the aprotic environment.
A co-formulation of insulin and pramlintide (an amylin analogue) – XP-3924 – is presently in clinical development, along with several other products. Figures 4 provides information on the efficacy and pharmacokinetics of glucagon formulated in this system, as well as some early clinical results from the insulin-pramlintide development effort. It is important to note that insulin and pramlintide would not be compatible in an aqueous formulation due to their differing pH requirements for solubility.
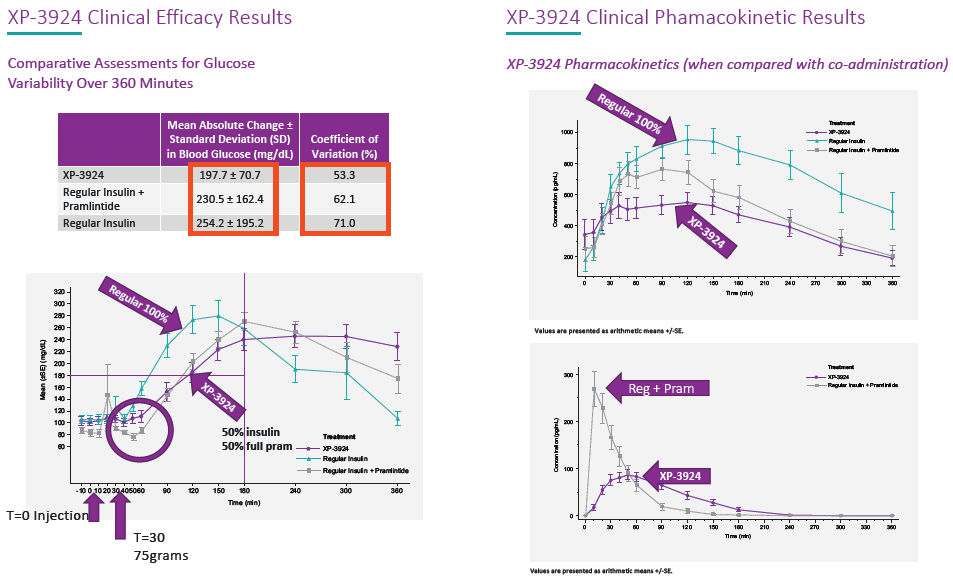
Figure 4: Pharmacokinetics of a Xerisol formulation of insulin + pramlintide.
To summarise, XeriSol enables peptide drugs to be developed, manufactured and marketed more rapidly and more efficiently – and at low risk of clinical failure due to formulation effects – all while achieving lower manufacturing cost, high efficacy and stability, greater packaging and presentation flexibility, broad compatibility with existing manufacturing equipment and compatibility with a wide array of advanced patient-friendly parenteral delivery devices that ensure precise dosing. The technology has been proven safe and effective in clinical trials and has received regulatory approvals globally.
XeriSol technology is indeed an elegant solution, and a potential paradigm-changing enabling technology for peptide therapeutics.
REFERENCE
- “Peptide Therapeutics Market – Growth, Trends, covid-19 Impact, and Forecasts (2022 – 2027)”. Mordor Intelligence, 2021.

