To Issue 173
Citation: Jomini T, Baronnet S, van den Anker J, “Enhancing Personalised Medicine: POWDOSE and Diffucaps for Precise Oral Solid Dosing”. ONdrugDelivery, Issue 173 (May/Jun 2025), pp 28–32.
Thierry Jomini, Stéphane Baronnet, Dr John van den Anker along with Giuseppe De Franza, evaluate the performance of the POWDOSE® system in delivering precise and repeatable doses of different formulations of Diffucaps®. The authors analyse the functionalities of combining Diffucaps with POWDOSE and highlighting its potential for improving personalised dosing regimens.
“THE POWDOSE DEVICE, A TWO-STEP MECHANISM, ALLOWS USERS TO FIRST PRESET THE DESIRED DOSE USING A RING AND THEN ACCURATELY DISPENSE THE MEDICATION BY TURNING A KNOB.”
Accurate dosing of oral solid forms is increasingly recognised as crucial for achieving optimal therapeutic outcomes while minimising adverse effects.1 Indeed, the era of the “one-tablet-fits-all” is in the past. Patients now seek personalised treatments for their individual needs. Tailoring drug doses will facilitate precise titration (e.g. in neurological diseases, ADHD2 and cardiovascular diseases3), help mitigate the development of antibiotic resistance4 and enable weight-based dosing, particularly in paediatric populations.5 Such individualised approaches are therefore critical for enhancing the safety, efficacy and overall effectiveness of treatment regimens.

Figure 1: POWDOSE device
AbbatiaLabs has developed a novel dosing technology that enables precise dose titration for oral solid medications by the end user. The POWDOSE device, a two-step mechanism, allows users to first preset the desired dose using a ring and then accurately dispense the medication by turning a knob. This user-centric design is optimised for ease of use and aims to minimise the risk of dosing errors, enhancing the reliability and safety of self-administered treatments (Figure 1).
Adare Pharma Solutions’ Diffucaps technology is a multiparticulate system that uses polymer membranes to create multilayered beads, allowing precise drug release and enhanced solubility in targeted gastrointestinal regions. Diffucaps can minimise side effects, such as gastric irritation, and reduce the impact of food on absorption.
This article evaluates and assesses the performance of the POWDOSE system in delivering precise and repeatable doses of different formulations of Diffucaps, demonstrating the system’s capacity to achieve consistent and reproducible dosing.
TEST MATERIAL
The POWDOSE device can deliver incremental doses constituting a fraction of the maximum deliverable amount. The technology is fully customisable, allowing adjustment of both the number of increments and the maximum dose to suit the specific requirements of the administered medication. In this study, four (1–4) POWDOSE devices were manufactured and tested. Each device was evaluated for its ability to deliver four distinct dose sizes, corresponding to 25%, 50%, 75% and 100% of the maximum deliverable dose. The reservoir was customised to accommodate up to 30 full-dose deliveries (Table 1).
| Dose Percentage (max) | Doses |
| 25% | 120 |
| 50% | 90 |
| 75% | 60 |
| 100% | 30 |
Table 1: Percentage of max delivered dose for this device configuration with the corresponding number of doses delivered.
With the flexibility needed to meet diverse patient requirements, Adare’s Diffucaps can be used in multiple dosage forms, including capsules, orally disintegrating tablets, rapidly disintegrating tablets and sprinkle formulations. When combined with other Adare technologies, Diffucaps can enhance solubility, broadening its applicability across various drug compounds and therapeutic areas.
For the purpose of this study, Diffucaps sugar sphere core granules were used for testing. These granules did not contain any API, meaning that no specific therapeutic dose was targeted or considered during the testing. As a result, the mean delivered dose for each dosing series was not compared with a predefined or target delivered dose. Instead, the study focused on evaluating the device’s ability to consistently deliver precise and reproducible increments of the granules across the specified dose fractions. This approach allowed for a comprehensive assessment of the device’s performance in terms of dose precision, independent of any therapeutic dose considerations:
- Devices #1, #2 and #3 were tested with Diffucaps sugar spheres size 30 LOT: 2310458 (sphere diameter between 500 and 700 μm).
- Device #4 was tested with Diffucaps sugar spheres size 18/20 LOT: 2410135 (sphere diameter between 800 and 1,000 μm).
TEST SETUP
The devices were fully loaded with the sugar spheres for the test. Each device was mounted on a laboratory stand positioned above a calibrated KERN F1 balance (capacity 240 g, precision 0.001 g). The test engineer operated the device per the test protocol, delivering the doses into a receptacle placed on the balance, which was used to measure the amount of material dispensed by the device during each test iteration.
The testing sequence for each device was as follows:
- 30 deliveries at the maximum dose
- 60 doses at 75% of the maximum dose
- 90 doses at 50% of the maximum dose
- 120 doses at 25% of the maximum dose.
Each delivery was logged in a test sheet.
METHODOLOGY
The objective of this study was to evaluate dose delivery variability by a device during its development, rather than to establish a quality control release testing protocol. A straightforward approach was employed, focusing on the total variability of the assay and the variability observed at different dosing stages (beginning, middle and end of the dosing process). As already described, placebo Diffucaps granules were used to assess the variability in the mass of doses dispensed by the device. Statistically, the relative standard deviation (RSD) is considered a practical acceptance criterion.
While the European Pharmacopoeia 2.9.40 on Uniformity of Dosage Units was referenced, the T value was not applied due to the absence of API in the granule batch tested. Instead, the limit of ±25% of the mean value was considered the critical parameter. Therefore, for the test to be considered successful, dose could not deviate by more than ±25% from the mean value.
The test was passed if the RSD was <7%, or if all doses were within ±25% of the mean for each device.
| % of Maximum Dose | Type of Diffucaps Granules | Devices Tested |
All Values Are Within ± 25% |
RSD | Target Value | Min and Max Values |
| 25 | Sugar spheres 500–700 μm | #1, #2, #3 | Yes | < 1.5% | 142 mg | 133 mg < x < 154 mg |
| 50 | Sugar spheres 500–700 μm | #1, #2, #3 | Yes | < 1.4% | 285 mg | 255 mg < x < 297 |
| 75 | Sugar spheres 500–700 μm | #1, #2, #3 | Yes | < 1.2% | 428 mg | 413 mg < x < 461 mg |
| 100 | Sugar spheres 500–700 μm | #1, #2, #3 | Yes | #1 < 0.75% #2 6.7% #3 < 0.75% |
570 mg | 558 mg < x < 585 mg #2 2 doses at 412 mg & 428 mg |
Table 2: Results for X% of the maximum delivered dose with devices #1, #2 and #3.
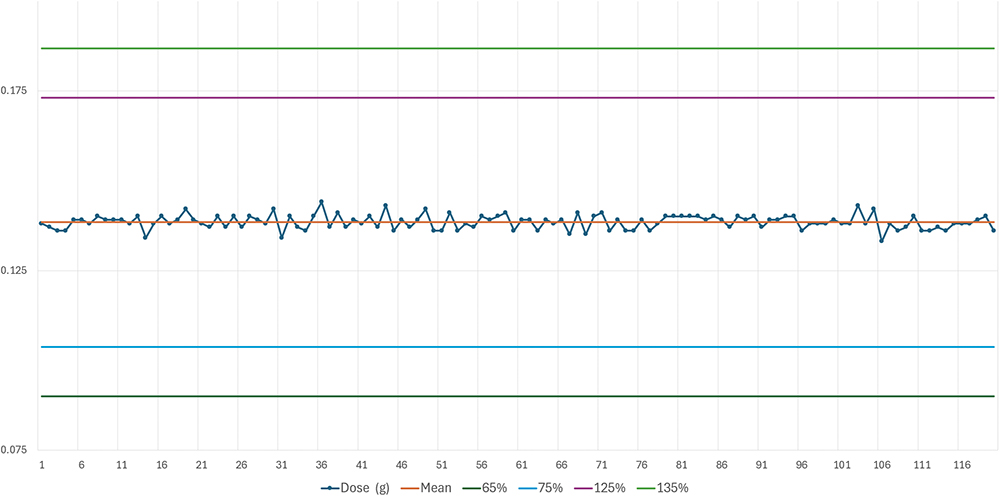
Figure 2: Delivered doses for targeted 25% of max dose with sugar spheres between 500 and 700 μm.
RESULTS
The results are summarised in Table 2. Figure 2 shows the delivered doses (g) targeted for 25% of the maximum dose with sugar spheres between 500 and 700 μm. Following the test with the sugar spheres 500 < x < 700 μm, another lot of Diffucaps sugar spheres was tested with a higher particle size: Diffucaps sugar spheres 800 < x < 1,000 μm. The results are shown in Table 3. Figure 3 shows the delivered doses targeted for 25% of the maximum dose with sugar spheres between 800 and 1,000 μm.
| % of Maximum Dose | Type of Diffucaps Granules | Devices Tested |
All Values Are Within ± 25% |
RSD | Target Value | Min and Max Values |
| 25 | Sugar spheres 800–1,000 μm | #4 | Yes | < 1.9% | 130 mg | 118 mg < x < 133 mg |
| 50 | Sugar spheres 800–1,000 μm | #4 | Yes | < 1.5% | 261 mg | 248 mg < x < 270 |
| 75 | Sugar spheres 800–1,000 μm | #4 | Yes | < 3.0% | 394 mg | 347 mg < x < 443 mg |
| 100 | Sugar spheres 800–1,000 μm | #4 | Yes | < 3.1% | 525 mg | 480 mg < x < 574 mg |
Table 3: Results for X% of the maximum delivered dose with device #4.
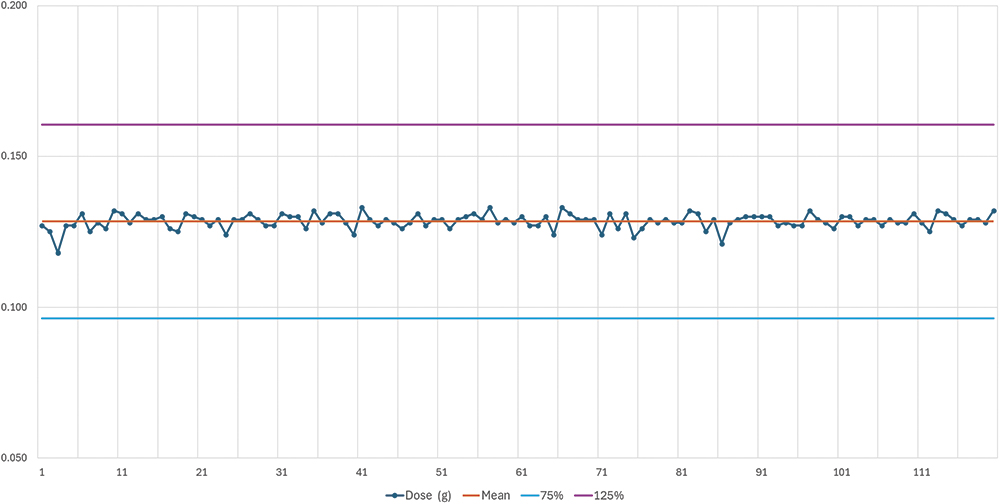
Figure 3: Delivered doses for targeted 25% of max dose with sugar spheres between 800 and 1,000 μm.
DISCUSSION
With 1,200 doses administered, the POWDOSE device demonstrated high precision and repeatability in delivering doses of Diffucaps sugar spheres. It is important to note that the devices used in this study were pre-production prototypes that have not yet undergone full industrialisation. As a result, the inner surfaces of the devices have not been optimised to minimise friction between the granules and the device walls. This optimisation will be addressed during the development and industrialisation process, which will occur prior to the product’s commercial release.
In typical usage, the POWDOSE device will be manipulated by the user between dose deliveries. This handling introduces slight shaking, which helps to prevent issues such as granule bridging, clogging, or sticking – phenomena that likely contributed to the two low doses observed in the fixed-device test setup.
The results also indicated that the doses remain consistent throughout the entire usage period of the device: 30–120 doses. No variation in dose delivery was observed, regardless of whether the dose was administered at the start of the device’s use (with a full reservoir) or at the end of its lifetime (when the reservoir was nearly empty).
Overall, the results indicate that Diffucaps granules in combination with the POWDOSE device provide a reliable and adequate solution for delivering customised doses of oral solid dosage forms. It provides a valuable tool for drug, chemistry, manufacturing and controls formulation teams, offering a means for patients to self-administer personalised doses of solid drugs with high accuracy and ease.
REFERENCES
- Singh BS, “The Impact of Pharmacogenomics in Personalized Medicine. Advances in Biochemical Engineering/biotechnology”. Adv Biochem Eng Biotechnol, 2020, Vol 171, pp 369–394.
- Farhat LC et al, “The effects of stimulant dose and dosing strategy on treatment outcomes in attention-deficit/hyperactivity disorder in children and adolescents: a meta-analysis”. Mol Psychiatry, 2022, Vol 27, pp 1562–1572.
- Mauriello A et al, “Pharmacogenomics of Cardiovascular Drugs for Atherothrombotic, Thromboembolic and Atherosclerotic Risk”. Genes, 2023, Vol 14, p 2057.
- Mouton JW et al, “The role of pharmacokinetics/pharmacodynamics in setting clinical MIC breakpoints: the EUCAST approach”. Clin Microbiol Infect, 2012, Vol 18(3), pp E37–E45.
- Bartelink IH et al, “Guidelines on paediatric dosing on the basis of developmental physiology and pharmacokinetic considerations”. Clin Pharmacokinet, 2006, Vol 45(11), pp 1077–1097.

