Citation: Canalejas L, “Formulation Solution for Moisture-Sensitive Drugs”. ONdrugDelivery, Issue 122 (Jul 2021), pp 28–31.
Laura Canalejas outlines the benefits of Quali-V® Extra Dry – a hypromellose capsule designed for moisture-sensitive formulations.
“The production process for Quali-V® Extra Dry was adapted to incorporate an extra drying phase by using unique equipment developed by Qualicaps Europe.”
It is well known that moisture affects the performance of some drug products, presenting a fierce challenge in the formulation of oral dosage forms. Moisture may have a significant impact on a wide range of chemical, physical and microbial properties of the finished pharmaceutical product, leading to a degradation of the API and a subsequent loss of potency and reduced shelf life.
The interaction of moisture with pharmaceutical solids is essential to an understanding of water-based processes, for example, manufacturing processes or prediction of the stability of solid dosage forms. Furthermore, both the API and excipients have different moisture sorption properties that can result in unexpected processing-induced phase transitions.1 Dosage forms are exposed to moisture from many sources – including bulk drug, excipients, manufacturing processes, environmental conditions, etc.2 In terms of excipients, using hard capsules could contribute to minimising the potential moisture effect on the formulation since they act as a container and therefore as a physical barrier to protect the filling content.
Back in the 1990s, Qualicaps introduced the first vegetal capsule based on hypromellose (HPMC) into the pharmaceutical market, branded as Quali-V®. This plant-based capsule made it possible to overcome stability issues that had emerged in gelatin capsules and were prevailing in the two-piece capsules market from the 19th century.
Despite the low moisture content present in HPMC capsules (three times reduced versus gelatin capsules), there are still some APIs (e.g. pancreatin, omeprazole, ranitidine, dabigatran and tiotropium) and excipients (polyethylene glycols, acid glycerol esters and acid triglycerides) whose hygroscopicity and sensitivity to moisture may alter the properties of the product.
Such hygroscopic and moisture-sensitive compounds could be challenging for formulators and may involve extra investment due to the need to apply different pathways to protect the API – i.e. galenic forms such as pellets or capsule-in-capsule technology, a final additional drying process after filling or the use of moisture-protective packaging materials. The possibility of drying the filled capsule to reduce the water content as much as possible exposes the whole filling to harsh drying conditions. Furthermore, adding an additional step in the process after the filling involves extra resources in terms of capabilities (with dedicated equipment, room, human resources and conditions) and timing, extending the process up to 12–14 hours and thus decreasing the overall yield.
“In HPMC capsules, water does not act as a plasticiser – hence the moisture content can be decreased whilst the mechanical strength remains.”
Quali-V® Extra Dry, the HPMC Capsule Designed for Moisture-Sensitive Formulations
Qualicaps, the pioneer hard capsule manufacturer, which developed thefirst HPMC capsule, has launched a novel HPMC capsule that resolves the aforementioned undesirable effects and additional costly countermeasures caused by moisture. Quali-V® Extra Dry is a capsule with an extremely reduced moisture content (2.0%–3.5%), half that of the standard HPMC capsules on the market.
In order to obtain such a low moisture value, the production process for Quali-V® Extra Dry was adapted to incorporate an extra drying phase by using unique equipment developed by Qualicaps Europe, with funding awarded by the European Union through the Centre for the Development of Industrial Technology. Nevertheless, this special drying process does not compromise the capsule’s physical or mechanical properties. The innovative capsule maintains the advantages of the standard Quali-V® – i.e. without preservatives, pharmaceutical grade, plant-based suitability for certain dietary and religious restrictions and chemically inert with the absence of crosslinking phenomena. Some Quali-V® Extra Dry properties are described hereafter and listed in Table 1.
| HPMC Extra Dry Capsule | ||||
| Parameter | Description | |||
| Moisture content | 2.0%–3.5% | |||
| Dissolution | USP and Eur.Ph dissolution profile at pH 1.2 and pH 6.8 | |||
| Brittleness | Minimal | |||
| Runnability | Tested in automatic high-speed filling machines: | |||
| MG2 Planeta 100 |
Bosch GKF-1400 |
IMA MATIC-120 |
IMA Adapta 100 |
|
| Storage conditions | 15°C–30°C in heat-sealed aluminium liners | |||
| Handling conditions |
Temperature: 20°C–30°C (set point 25°C ± 2) RH: 15%–25% (set point 20% ± 2) |
|||
| Shelf Life | Capsule: 18–24* months Final drug product: 2–3 years |
|||
* Stability studies carried out at ICH accelerated and long-term conditions meeting the specifications after 6 months.
Shelf life depends on the size of the capsule.
Table 1: Description of the Quali-V® Extra Dry capsule properties.
Brittleness is a critical aspect when capsules come into play. Water in gelatin capsules functions as a plasticiser and, when this level falls below the specification limits, the capsules become brittle and crack on handling. However, in HPMC capsules, water does not act as a plasticiser – hence the moisture content can be decreased whilst the mechanical strength remains.3,4,5
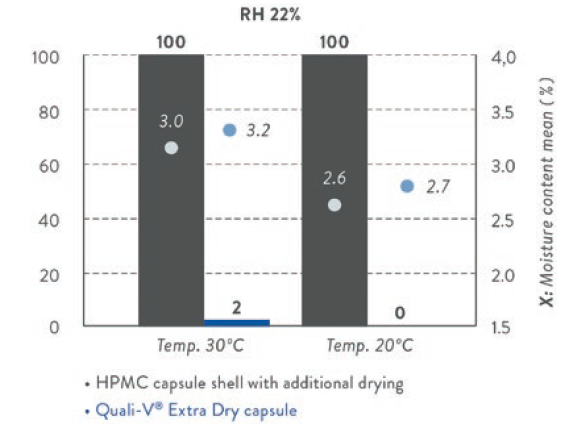
Figure 1: Capsule brittleness/impact test (empty capsule; n=50) (Qualicaps Japan).
An impact test, by dropping a weight of 50 g from 10 cm above the capsule, was performed to evaluate how fragile Quali-V® Extra Dry could be at low relative humidity environmental conditions: minimal brittleness has been observed, as shown in Figure 1.
The dissolution profile of this novel capsule is equivalent to the one of standard HPMC capsules, complying with both USP and Eur Ph dissolution profiles at either pH 1.2 or pH 6.8 (Figures 2 and 3).
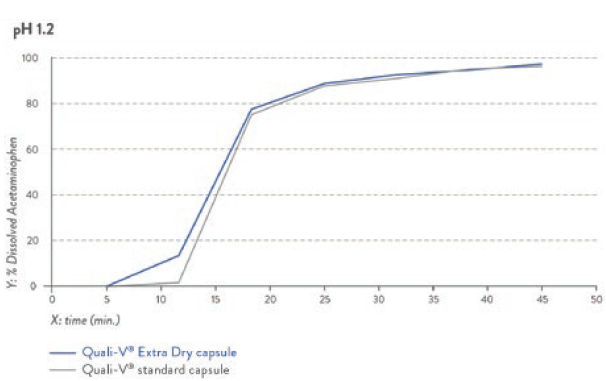
Figure 2: Dissolution profile for pH 2.1 capsule fill formulation – acetaminophen.
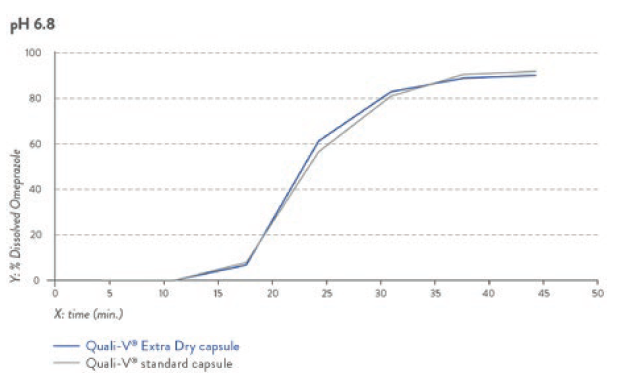
Figure 3: Dissolution profile for pH 6.8 capsule fill formulation – omeprazole pellets.
It is important to highlight that Quali-V® Extra Dry requires specific handling and storage conditions in order to retain the moisture content within the specification and thus secure its quality properties. Since Quali-V® Extra Dry is provided in heat-sealed and moisture-proof aluminium liners, only the temperature needs to be controlled between 15°C and 30°C when storing these capsules.
Once the bag is opened, the following handling and filling conditions are highly recommended: a temperature between 20°C and 30°C as well as a relative humidity in the range of 15%–25%. Such handling conditions have been defined by performing an absorption and desorption study with a volumetric method (Figure 4).
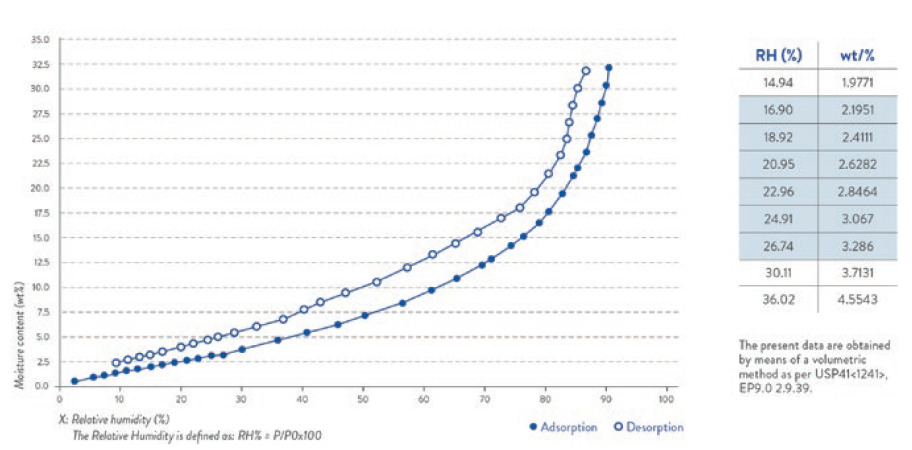
Figure 4: Water vapour absorption/desorption isotherm curve (25°C) (Qualicaps Japan).
The present data are obtained by means of a volumetric method, as per USP41<1241>, EP9.02.9.39.
CONCLUSION
In summary, the Quali-V® Extra Dry capsule, with a guaranteed extremely low moisture content, provides enormous benefits when formulating moisture-sensitive drugs. It has a number of advantages over standard HPMC capsules – such as superior chemical inertness, stability results by avoiding compatibility issues and brittleness when environmental conditions are out of the standard range – and the Extra Dry capsule makes it possible to maximise those benefits even when formulating moisture-sensitive drugs. Furthermore, the Quali-V® Extra Dry capsule may limit or even avoid the need for other measures to control moisture, such as adding a drying step in the filling process, thus improving the manufacturing yield.
REFERENCES
- Airaksinen S et al, “Role of water in the physical stability of solid dosage formulations”. J Pharm Sci, Oct 2005, Vol 94(10), 2147–2165.
- Gerhardt AH, “Moisture Effects on Solid Dosage Forms – Formulation, Processing and Stability”. J GXP Compliance, Jan 2009, Vol 13, pp 58–66.
- Ogura T et al, “HPMC capsules – An alternative to gelatin”. Tech Europe, Nov 1998, article number 0310, p 5.
- Nagata S, “Advantages to HPMC capsules – A new generation’s hard capsules”. Drug Deliv Technology, Jan 2002, Vol 2(2), pp 1–5.
- Jones BE, “A new solution for formulation challenges”. Business Briefings, Pharma Outsourcing, Jan 2004, pp 56–61.

