Citation: Müller M, “High-Speed Assembly of Needle Shields for Prefilled Syringes”. ONdrugDelivery, Issue 178 (Oct 2025), pp 102–105.
Matthias Müller discusses the factors that set exceptional manufacturing equipment apart from the rest and how Contexo‘s new machine for assembling needle shields with prefilled syringes meets all these expectations.
“THE CHALLENGE IS TO BUILD A MACHINE WITH A SMALL FOOTPRINT THAT IS EFFECTIVE, RELIABLE AND FAST, AND CONTEXO CONSISTENTLY RISES TO THE OCCASION.”
Over 25 machines leave Contexo’s production facilities every year. The company’s medical device business is especially important and is growing continually. There is good reason for this – Contexo’s decade-long experience of building highly efficient machines, coupled with its inventiveness, produces assembly solutions that satisfy even the toughest requirements while remaining fairly priced. “Everything in-house” is the Contexo way. The challenge is to build a machine with a small footprint that is effective, reliable and fast, and Contexo consistently rises to the occasion.
CONTINUOUS MOTION TECHNOLOGY
As the demand for prefilled syringes continues to rise, so does the need for needle shields. Contexo is currently developing a continuous-motion machine for assembling needle shields as part of its commitment to providing the medical sector with fast and reliable solutions. Working with these products requires in-depth knowledge and experience. Contexo has developed a standard platform on which assembly machines for rigid or soft needle shields can be built quickly and efficiently. The core of this system is the continuous motion technology (Figure 1), which assembles and crimps using two central towers. This enables the system to supply large quantities using stable, reliable processes.

Figure 1: Continuous motion assembly machines.
“CONTEXO HAS DEVELOPED A STANDARD PLATFORM ON WHICH ASSEMBLY MACHINES FOR RIGID OR SOFT NEEDLE SHIELDS CAN BE BUILT QUICKLY AND EFFICIENTLY.”
It is essential that the process is well controlled, robust and repeatable. After the cover and needle shield have been fed in smoothly, both parts are pressed and passed on to the thermoforming tower. Since the crimping of the needle shields is critical, this process must meet the highest standards. Consequently, the machine has been designed so that each temperature value in the thermoforming tower can be set individually (Figure 2). This key process is constantly monitored.
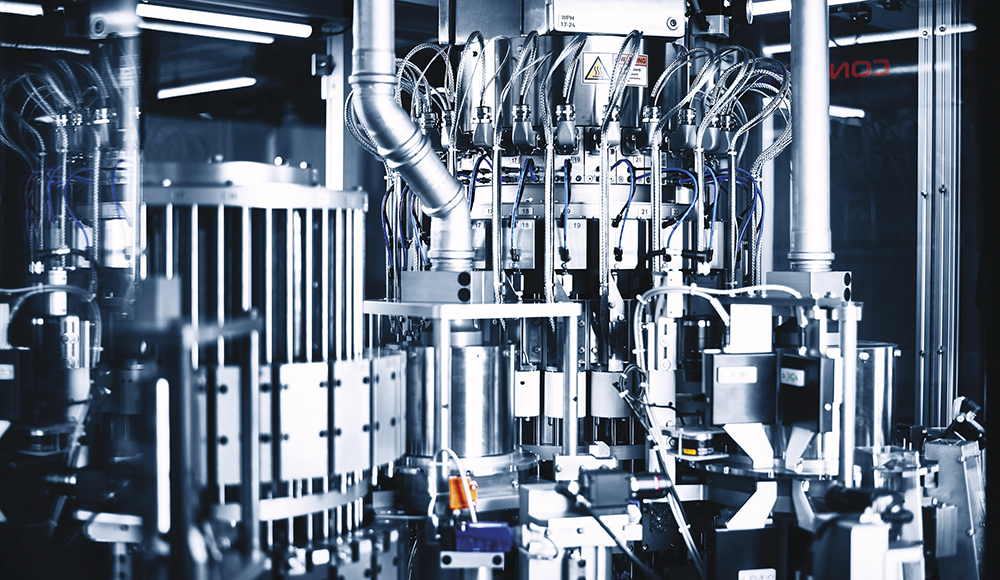
Figure 2: Thermoforming tower.
Quality control is performed 100% in-line via visual inspection; three cameras perform the various tests (Figure 3). At the end of the process, all product-relevant features are checked again using open and transmitted light controls, and the parts are separated into good and bad. Contact-free inspection and control systems ensure zero contamination.

Figure 3: 100% in-line vision inspection – zero defects.
LONG LIFESPAN
The machine is designed for long-term use and has been built to be robust from the ground up. The use of mechanical components guarantees years of optimal performance and an almost fault-free system. This results in low maintenance requirements and a long service life. The components are made of stainless steel and have been encapsulated to facilitate hygienic cleanroom production (Figure 4).
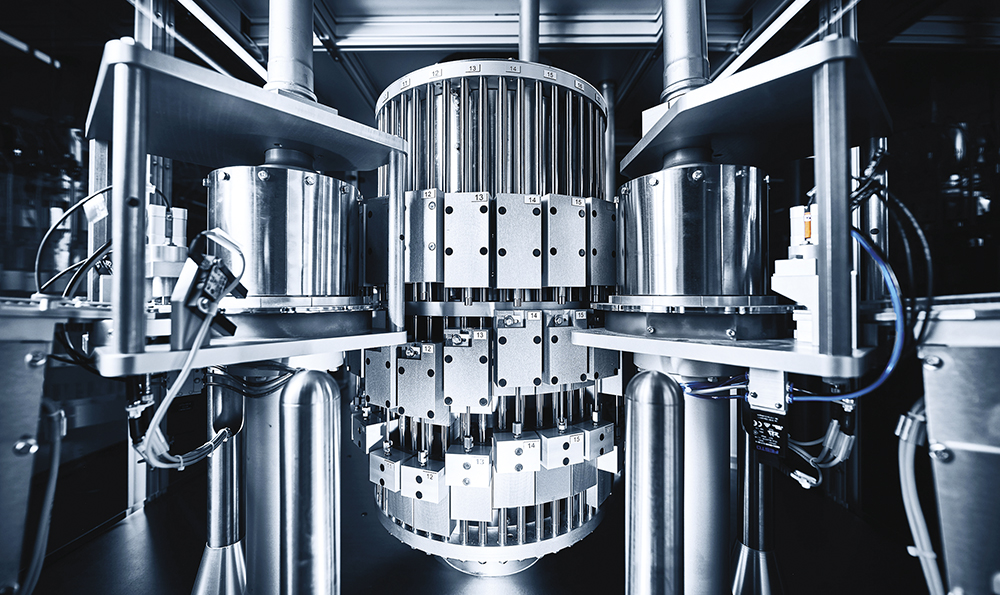
Figure 4: Stainless steel cleanroom production.
“WHEN MANUFACTURING IN CLEANROOM CONDITIONS, IT IS ESSENTIAL FOR PRODUCTION TO REMAIN COST-EFFECTIVE WHILE ALSO FULLY COMPLYING WITH THE RELEVANT REGULATIONS.”
Operation, maintenance and care have been incorporated into the design from the outset. All surfaces are easy to clean and readily accessible. The space-saving machine concept minimises costs (Figure 5). When manufacturing in cleanroom conditions, it is essential for production to remain cost-effective while also fully complying with the relevant regulations. In addition to the minimal footprint, it is crucial for the system to be flexible. In line with this requirement, the machine can be easily adjusted to new requirements and be flexibly adapted to meet the needs of different products.

Figure 5: High-speed assembly.
“CONTEXO OFFERS VARIOUS SOLUTIONS THAT ARE ALL IN ACCORDANCE WITH CURRENT INTERNATIONAL STANDARDS
AND PROVIDES A COMPREHENSIVE GMP PACKAGE, INCLUDING LOG GENERATION, TEST EXECUTION AND DOCUMENTATION OF RESULTS.”
FULL GMP QUALIFICATION
It is mandatory for machines in the medical industry to be qualified. Contexo offers comprehensive and up-to-date GMP compliance. GMP qualifications for production, assembly or quality control processes require an expanded GMP-compliant qualification document to be created. Contexo offers various solutions that are all in accordance with current international standards and provides a comprehensive GMP package, including log generation, test execution and documentation of results.
SHORT PROJECT TIMES
In addition to its reliable technology, Contexo impresses with its short project times. Contexo is well prepared and provides companies with its full range of experience – it is ready to go and its products can be used very quickly. Manufacturers benefit from rapid, professional implementation, enabling them to market and supply their products swiftly.

