Citation: An R, Harding-Moore G, “How to Develop a Customised Drug Delivery IoT Platform with a Running Start”. ONdrugDelivery Magazine, Issue 103 (Dec 2019), pp 22-25.
Do you need a drug delivery Internet of Things (IoT) platform across your entire portfolio of drugs that is agnostic to the type of source container, drug viscosity and the volume to be dispensed? Rachel An and Gillian Harding-Moore discuss how to develop such a platform with a running start.
The market for connected drug delivery devices is predicted to grow at an exponential rate over the next 5-6 years.1 The obstacles to adoption are centred around data security, patient safety and regulation – but the potential benefits for pharma companies, patients, payers, doctors and hospitals are unquestionable, and therefore the industry is beginning to make this technological leap forward. Drug companies are recognising that it will be essential to provide connectivity in order to remain competitive, so we predict that, by 2030, connected drug delivery devices will be regarded as the norm.
“Pharma companies are now faced with the daunting task of creating connected devices that are customised to their specific drugs or specific range of drugs.”
One of the main benefits of using connected devices will be improvement of – and insight into – patient adherence. The effectiveness of any medicine is impossible to measure without knowing whether a patient is, in fact, taking the medicine. The consequences of poor adherence to long-term therapies are poor health outcomes and increased healthcare costs. But current methods for measuring adherence are usually subjective and remain only an estimate of patients’ actual behaviour.2
Smart drug delivery devices not only provide immediate feedback to the user on how and when to take their medication but also track what treatments the patient has taken. Big data insights are invaluable for companies to gather patient feedback, habits, indications and side effects, providing significant opportunities for product improvement.
There is a rise in demand for home treatment, driven by a growing ageing patient population, an increase in the prevalence of chronic conditions and patient preference. The ability to monitor patients’ home infusions remotely will also free-up general practices and hospitals – saving healthcare costs. Finding ways for patients to use one device that can be used throughout treatment in hospital, then taken home with them to continue treatment is far more cost effective and easier for the patient to learn how to use. It also provides reassurance that the device used in the hospital is the very same as the device taken home.
It is difficult and costly to track and retrieve expensive equipment once it leaves the hospital, so less expensive solutions are in demand. Spending less time in hospitals and more time self-administering at home is far more comfortable for patients, who will also largely be assured by the knowledge that their progress is being monitored by a healthcare professional in real time.
So pharma companies are now faced with the daunting task of creating connected devices that are customised to their specific drugs or specific range of drugs. Fortunately, innovation is happening in this space, and more and more connected devices are launching into the market for pharma companies to adopt for their purposes.
THE PROBLEM WITH OFF-THE-SHELF CONNECTED DEVICES
“Building a custom pump solution from scratch is a huge financial and resource-time risk.”
Connected devices available today can provide neat solutions that have been tested and regulated. Using an off-the-shelf device means a product can be launched to market relatively quickly. However, these devices tend to be designed for a wide range of applications, with multiple features that cover the requirements of their range of compatible conditions. This means they often contain too many options – which can be confusing to the patient because they are not all required by their specific treatment, which can lead to misuse or user errors.
Building a custom pump solution from scratch is, however, a huge financial and resource-time risk, as it will require a large focused team and colossal financial investment. So, is there a way to mitigate that risk and ensure you can create a perfectly bespoke solution, based on your requirements, that you can still take to market relatively quickly and will lead to an early win?
The answer today is YES… you develop and customise an existing drug-delivery IoT reference platform that is driven by a flexible and unique precision pump technology.
CONNECTED DRUG DELIVERY REFERENCE PLATFORM
Before we talk about the platform, we need to introduce its most important and unique element – the Quantex precision pumps, which we like refer to as the “Intel Inside”. These patented pumps use a positive displacement rotary action to carry a fixed volume of fluid from inlet to outlet. This unique pumping mechanism allows for accurate dosing without any software calibration, even when pumping viscous fluids against high back pressures. The pump generates enough vacuum pressure to pump from bags, syringes and vials and is not sensitive to head height.
All of this is achieved with one moving part, which means it is reliable, low-cost and easy to control. Because they are so inexpensive to manufacture, the pumps are single use and can be integrated in-line with the disposable drug pack and infusion set, which means no maintenance or calibration is required. The CS-3 and CS-6 micropumps (Figure 1) are standard medical pumps within the Quantex portfolio. These two pumps will infuse anything from doses of 1 uL up to flow rates of 2.4 L/hr.
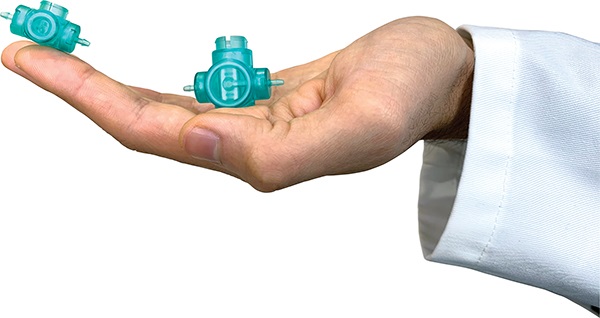
Figure 1: Quantex CS-3 and CS-6 micropumps.
Due to these properties, Quantex pumps allow for flexibility in the design of an integrated system. Fittings on the inlet and outlet of the pump can either be standard (luer, hose barb) or custom fittings, allowing for easy integration of the pump in-line. The simple rotary action of the Quantex pumps means flow is easily controlled with a motor: the speed of the motor controls the flow rate, and the number of revolutions determines the volume pumped. Furthermore, the pumps have a low torque requirement which means the device can readily be battery powered. Since the information required to control the pump is simple, different wireless communication methods can be used to transfer information to and from the device.
“Information on any logged infusion events, adherence and patient experience can be gathered to personalise treatments for greater effectiveness.”
QUANTEX 4C
To showcase the possibilities of integrating the Quantex pumps into a drug delivery system, Quantex has developed an IoT reference platform that takes advantage of the pumps’ unique features. The Quantex 4C system consists of a Quantex single-use pump and re-usable pump driver device. A smartphone application is used to program the device and report information back from the device. Real-time infusion data is relayed to a web portal, where a doctor or other healthcare professional can remotely monitor their patients’ infusions.
Information on any logged infusion events, adherence and patient experience can be gathered to personalise treatments for greater effectiveness. Appropriately depersonalised data can be used by companies for valuable business insight and post-market surveillance, as well as by insurance companies for value-based contracting.
There are currently three devices in the Quantex 4C family (Figure 2), all with different capabilities and features to show a range of what is possible:
- Quantex 4C: This device has been designed for the single-use CS-3 pump and can dose at a rate up to 110 mL/hr. The device uses wireless charging which allows for a fully sealed, waterproof design and can pump up to 100 mL on a single charge
- Quantex 4C Lite: This device is so inexpensive to manufacture that both the CS-3 pump and driver device can be considered single use and would typically be used in emergency situations. It can pump up to 10 mL at flow rates up to 0.5 mL/hr
- Quantex 4C Hi-Flo: This device has been developed for the larger CS-6 pump and can reach flow rates of 2.4 L/hr.
MODES OF COMMUNICATION
Three different modes of wireless connectivity have been incorporated into the Quantex 4C system. Near-field communication (NFC), as the name suggests, is a short-range wireless connectivity standard. This technology is used in contactless card payments, where magnetic field induction enables communication between devices placed in close proximity (a few centimetres). NFC is intuitive and does not require a search-and-pair procedure like other methods to establish connectivity.
The Quantex 4C system uses NFC communication both to program an infusion profile (drug information, flow rate, volume, etc) and also report information from the device, with just the tap of a smartphone. Its high level of encryption, as well as the need for the phone to be physically held near the device, offers security benefits.
Bluetooth Low Energy (BLE) is a longer-range wireless connectivity standard with minimal power consumption. BLE allows for real-time feedback from the device to the smartphone, as well as the integration of other peripheral devices or sensors into the system. The Quantex 4C smartphone application uses BLE to display current status of infusion from the device, as well as send commands from the phone to pause or resume the infusion.
The Quantex 4C platform has integrated a flow sensor by Sensirion that monitors the flow of the infusion near the injection site. This information is sent via BLE to the smartphone, allowing for detection of occlusion and air-in-line. This means there is an independent method of confirming the flow rate and total dose into the body, with the added benefit of allowing self-priming without needing calibrated lines.
Internet connectivity (4G, Wi-Fi, NB-IoT, etc) is used to transfer infusion data gathered from the devices to a web portal. This opens the range of connectivity of the system to anywhere that has internet access. Doctors can monitor their patients’ infusions, as well as send prescriptions and infusion profiles remotely.
Determining which mode of communication is most appropriate for each type of data transfer (doctor to device, device to doctor, device to patient, etc.) will largely be based on the application or use case. The Quantex 4C system has flexibility on which method(s) of connectivity are used, allowing flexibility in developing the best workflow and system architecture for all stakeholders.

Figure 2: The Quantex 4C range of devices – all compact enough to fit in the palm of your hand.
FLEXIBILITY AND CUSTOMISABILITY OF A REFERENCE PLATFORM
The potential benefits of connected medical devices for patients, pharma companies,payers and healthcare professionals are undeniable – improving adherence to treatments, freeing up hospitals and big data insight will bring great returns to those who adopt connected device systems, as long as they “do it right”. There is already increased adoption of IoT, patient connectivity and engagement – the trend is rising but it needs careful execution.
Adding connectivity and dedicated apps to support specific therapies has never been easier and, with the rapid onset of new wireless technologies, it’s important to also have a pump technology that is able to take full advantage of all this potential. Quantex aims to make it easier for companies to enter this exciting new world of connected devices by providing a foundation on which to build their custom connected systems with Quantex pump technology at their core (Figure 3).
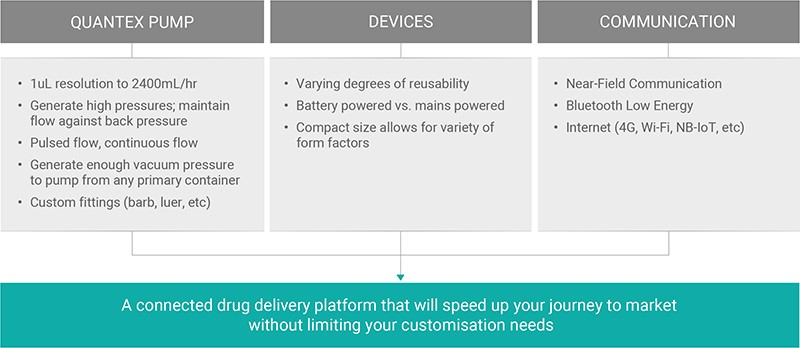
Figure 3: A summary of the flexibility and customisability of the Quantex 4C drug delivery IoT platform.
REFERENCES
- “Connected Drug Delivery Devices Market Worth $717.7 Million By 2025”. Grand View Research, 2018.
- “Adherence to long-term therapies: evidence for action”. World Health Organization, 2003.

