Citation: Schneider A, “I Spy With my Little Eye: Eye Tracking Drives Patient-Centred Development of Connected Systems”. ONdrugDelivery Magazine, Issue 98 (Jun 2019), pp 44-47.
The development of connected self-injection systems is driving industry to rethink its current approaches to patient-centred new product development. Andreas Schneider summarises a recent field study that used eye tracking for the formative usability testing of SmartPilot for YpsoMate – a re-usable passive monitoring module that transforms the two-step autoinjector YpsoMate into a fully connected product.
Patient centricity has long been regarded as a guiding paradigm for the development of self-injection devices. Industry and academia alike have intensively studied how to capture the patient’s voice optimally throughout the innovation process – either during early-stage formative usability testing or as part of late-stage summative design validation.
“The development of connected self-injection systems is about to challenge the well-established methodological principles.”
Interestingly, there has been little methodological advancement todate. For three decades or so, the industry has largely relied on patient self-reports, open-ended interviews or shadowing users during their daily drug administration routines as design inputs. Such insights led to innovative self-injection device concepts. In fact, these approaches have built a standard methods usability toolbox that – if adequately applied – permits patient-centred product development.
Entering the digital era, that is no longer true. The development of connected self-injection systems is about to challenge the well-established methodological principles.
Connected self-injection systems typically consist of multiple components and user interface elements that may advance patient adherence to the next level. However, the interplay between these elements – such as an autoinjector, a companion mobile application and a re-usable connected monitoring module (Figure 1) – must be carefully orchestrated. How to guide users step by step through the injection process? Where to locate visual, audible or tactile feedback elements? How to blend the mechanical with digital elements?
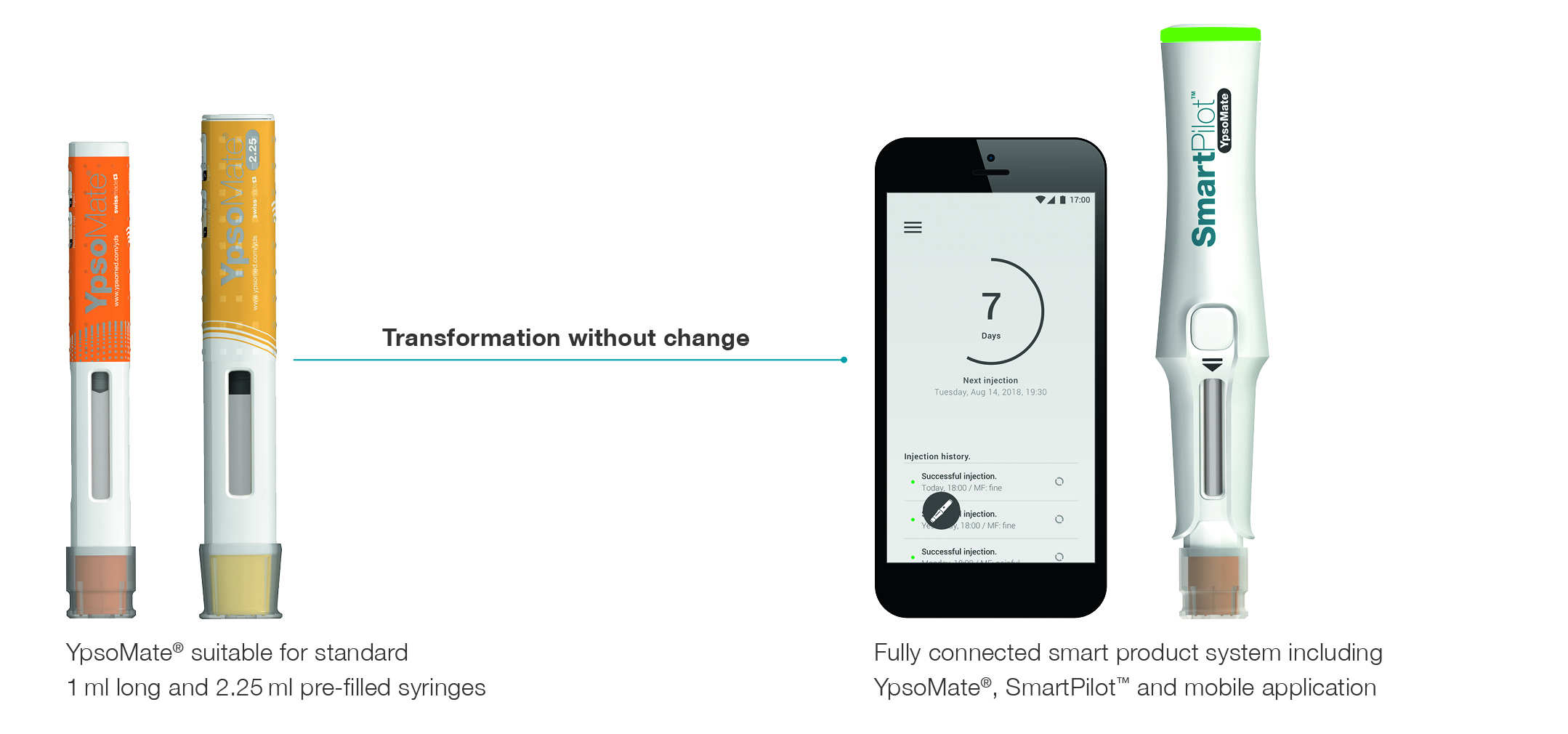
Figure 1: The connected self-injection device system included in the study.
And yet, patients may not be able to appropriately verbalise their experiences when operating such a multi-element product system. Knowledge in general is hard to put into words. Verbalising experiences becomes particularly challenging when tied to emotions, intuition or physical experiences. New usability approaches are thus needed to inform the design development of connected self-injection device systems better.
A recent study investigated the application of eye-tracking in the development of connected self-injection systems.1 Figure 1 illustrates the need to optimise the interplay between user interface elements of any connected self-injection device system.It introduces the study device: SmartPilot for YpsoMate. SmartPilot is a re-usable smart monitoring module that flexibly transforms the marketed YpsoMate autoinjector into a cloud-connected product system. Advanced sensors track injection events and guide patients in real time through the injection process. Therapy-relevant injection data is encrypted on-chip and then made available to the broader healthcare ecosystem via wireless connectivity. Thus, YpsoMate comes with two sets of key functionalities. One monitors device usage and makes available the therapy-relevant injection data to providers, caregivers and healthcare stakeholders. The other guides patients step-by-step through the drug administration process including, for example, authentication of the self-injection device, or advice on holding time.

Figure 2: A snapshot of the experimental set-up – image A shows the use of eye-tracking glasses while image B illustrates the study participant’s visual attention as an orange ring.
AN INTRODUCTION TO EYE TRACKING
Neurologists have long concluded it is through seeing that we construct meaning. Tracking patients’ gaze patterns thus sheds light on their cognitive attention. Mobile eye tracking continually follows participants’ eye movements as they engage with a connected self-injection system. The gaze patterns collected serve as input to evaluate the underlying cognitive processes during user engagement with a product system. The method thus permits the objective assessment of product usability without the need for participants to express their experiences while performing use steps.
Figure 2 illustrates the study’s experimental set-up and the type of raw data obtained through mobile eye tracking. The evaluation required the use of eye-tracking glasses (Figure 2A), which must allow participants to move without restrictions while engaging with the product system. In this study, the SMI Eye-Tracking Glasses 2 Wireless system (SensoMotoric Instruments, Teltow, Germany) was used. Cameras integrated in the glasses capture the participant’s field of view and construct the “scene video”. The data are then converted into the viewing angle and the participant’s continuous gaze point. Figure 2B shows the study participant’s visual attention represented as an orange ring. The tracked gaze patterns served as a starting point for evaluation.
Figure 3 shows the research design of the simulated use study. Three eye-tracking studies were conducted, each informing a subsequent design iteration of the connected self-injection system’s user interface. A total of 35 injection-naïve participants were newly recruited for the studies, during which participants simulated a self-injection into a foam cushion. The users were not exposed to the connected product system prior to the study. The companion mobile application installed on the smartphone provided real-time step-by-step instructions on how to operate SmartPilot for YpsoMate.
The insights gained from each eye-tracking study then informed the subsequent design iteration of the connected self-injection system. Three different design variants were evaluated, providing a longitudinal perspective on how product usability has evolved over time.
EYE TRACKING EVALUATED THREE USABILITY DIMENSIONS
The results of the empirical study concluded that eye tracking effectively guided the evolution of product usability along three dimensions – effectiveness, efficiency and ease of use. These usability indicators, as derived from eye tracking, improved as a result of the modifications and design adjustments made to the connected self-injection system. Figure 4 summarises the key findings of the empirical study.The study provides empirical evidence that the overall product usability was step-wise improved over the course of the three eye-tracking experiments (ETS1, ETS2 and ETS3).
First, the data confirms that the product usability dimension, effectiveness, improved substantially over time. Effectiveness describes the user’s ability to complete a certain use-step successfully. The extent to which a use-step was successfully completed was assessed using a four-point rating scale with the following items: (1) use error requiring moderator intervention; (2) close call/ temporary interruption; (3) user hesitation; and (4) uninterrupted completion.
Eye tracking provided rich contextual data from a unique first-person perspective to capture that first dimension. Detailed analysis of the video data recorded using the eye-tracking glasses shows that not only were use errors significantly reduced but also the overall robustness of the product increased. Initial usability challenges that resulted in process interruptions or use errors were completely eliminated over time.
Second, formative eye-tracking data also showed a step-wise improvement of the usability dimension efficiency, meaning a reduction in the duration needed to complete a specific use step. Shorter visual attention per user interface element corresponds with lower cognitive efforts to understand product usage. Eye-tracking data enabled the separation of overall product usage into distinct use steps and the precise definition of the transition points between them. The results confirmed a step-wise decrease in the duration required to complete the overall use process, thus providing empirical evidence that overall product efficiency was improved.
“User guidance was improved as a result of the design iterations guided by the eye-tracking studies.”
Third, eye-tracking-derived indicators also shed light on ease of use. Here, the product system is dissected into separate user interface elements. This shows how users directed their visual attention to those elements that were required for the completion of the given use step. The study advanced our understanding that eye-tracking data can be used to capture ease-of-use data objectively for each user-interface element. The results showed that user guidance was improved as a result of the design iterations guided by the eye-tracking studies: the more mature the connected self-injection system became, the less visual attention – and thus cognitive effort – was lost on irrelevant user interface elements.
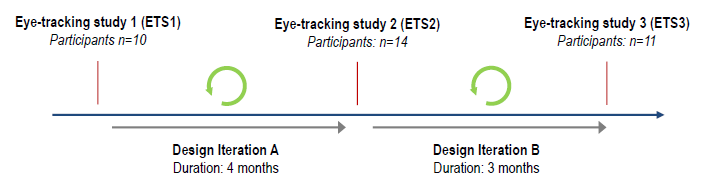
Figure 3: The longitudinal research design of the empirical study. Injection-naive users were recruited anew for each study.
PATIENT CENTRICITY – IN THE EYE OF THE BEHOLDER?
The study summarised here shows how eye tracking enables an in-depth analysis of patient engagement with a connected self-injection system, with a level of detail that would have gone unnoticed using traditional methodologies. Eye tracking confirmed that the design iterations targeting the usability of the connected self injection system SmartPilot for YpsoMate were highly effective. Specifically, the eye-tracking-derived indicators were of pivotal importance to assess the improvement of three usability dimensions over time: effectiveness, efficiency and ease of use. Empirical evidence tells us that the interplay between the distinct user interface elements was improved as the system’s maturity increased.
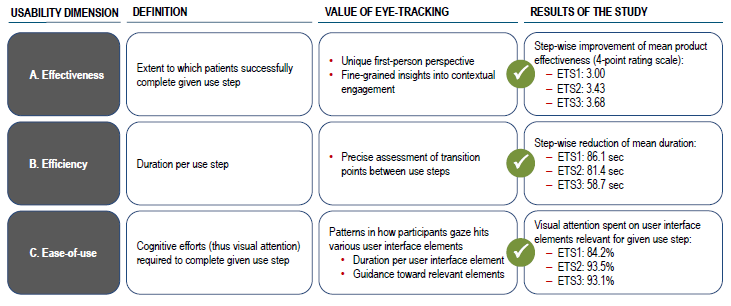
Figure 4: Overview of the results of the empirical study (ETS = eye-tracking study).
The results furthermore suggest that eye tracking has great potential to guide new product development beyond connected self-injection systems. The digital transformation will radically change the way in which users interact with digital tools and connected technologies. In particular, the digital era drives the adoption of software-enhanced multi-element product systems – such as smart inhalers, sensor-augmented smart insulin pump systems or smartphone-based mobile electrocardiogram (ECG) devices – both at home and in the critical care environment. These systems all share a common trait: the digital and mechanical elements become integrated to realise advanced user feedback.
The relevance of eye tracking thus continues to grow. Formative and summative usability testing will increasingly study how to orchestrate the interplay between distinct user interface elements. Hence the drive to adopt novel methodological approaches to usability testing. New product development must result in the marketing of innovative healthcare tools and technologies that improve ease of use, rather than increasing patient cognitive burden.
REFERENCE
- Lohmeyer Q et al, “Toward anew age of patient centricity? The application of eye-tracking to the development of connected self-injection systems”. Expert Opin Drug Deliv, 2019, Vol 16(2), pp 163-175.

