To Issue 177
Andersen B, Simpson I, “Improving the Self-Administration of Lyo- and Liquid-Liquid Formulations”. ONdrugDelivery, Issue 177 (Sep/Oct 2025), pp 72–78.
Bjørn Andersen and Iain Simpson explore the factors contributing to interest in lyo-liquid and liquid-liquid drug delivery, highlighting some of the current unmet needs in this space. Following this, they discuss how platform technologies, such as electromechanical drives, may help address these unmet needs and improve the stability and convenience of administering lyophilised and dual-liquid formulations from dual-chamber containers.
Many modern therapeutics – particularly biologics (such as proteins, monoclonal antibodies, peptides, vaccines and enzymes) – are inherently unstable in aqueous solutions. Lyophilisation is an established method used to address this challenge. By removing water under low temperature and vacuum, the process locks the active ingredient into a dry matrix that reduces chemical and physical degradation.
A 2023 review of approved biotherapeutics identified 89 marketed antibodies in a total of 96 presentations of which 22 (22.9%) were presented as lyophilised powders.1 An analysis of PharmaCircle data by Phillips Medisize yielded 71 lyophilised products approved for subcutaneous delivery between 2014 and 2024 inclusive (Figure 1). Several factors seem to be involved:
- Some biologic drugs are inherently unstable. For example, messenger RNA (mRNA)-based and cell and gene therapies often require storage at ultra-low temperatures – sometimes as low as -70°C. These storage requirements can present challenges for distribution logistics. Lyophilisation may offer a way to store and transport these drugs at higher temperatures.2
- Lyophilisation may allow for higher concentrations of injectable drug formulations, which could support subcutaneous delivery as single injections instead of higher-volume infusions.3
- Compared with developing liquid-stable formulations, lyophilisation may offer a faster route to drug product development for clinical studies and initial market entry. Liquid-stable formulations typically require investigation and selection of excipients to address potential issues, such as aggregation, oxidation or hydrolysis over the product’s shelf life.4 Demonstrating adequate stability to support early clinical studies may be quicker for lyophilised formulations.
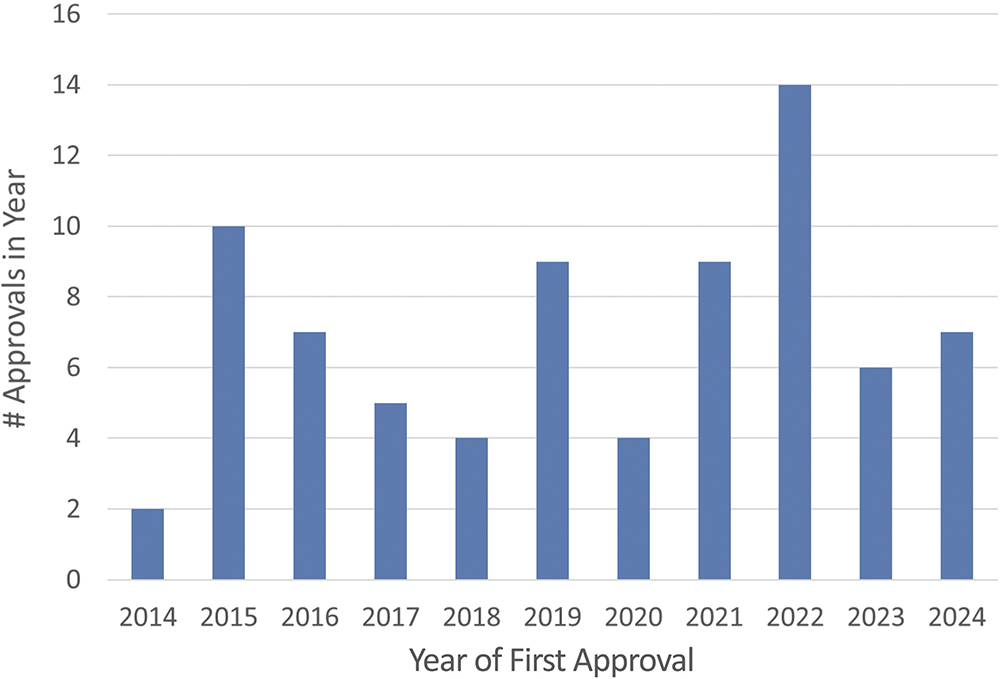
Figure 1: Marketed subcutaneously delivered lyophilised drug products by year of approval.
However, several challenges continue to influence the adoption of lyophilised drug formulations in commercial drug products. These include:
- Higher manufacturing costs associated with longer cycle times, capital expenditures for processing equipment and energy use during the lyophilisation process.
- Process complexity and potential scale-up challenges. Transferring lyophilisation processes from pilot to commercial scale may involve difficulties, and adjustments to the process could require additional revalidation.
- The need for reconstitution before injection places a burden on the user of the drug product. While this may be less of an issue for healthcare professional (HCP)-administered drugs, the shift towards patient and caregiver administration could increase the risk of use errors. In markets where lyophilised drugs might need to compete with liquid-stable formulations in easy-to-use devices, this factor may affect their uptake.
- Dual-chamber containers offer a good approach to reduce patient and HCP burden in preparing and administering lyophilised formulations. However, only a limited number of CMOs currently offer commercial scale filling services for dual-chamber primary containers.
Combining multiple therapies into a single dose – known as fixed-dose combinations (FDCs) – is an area of focus in drug development.5 Delivering FDCs as a single dose requires coformulation, which may pose challenges in ensuring long-term drug stability due to potential drug-drug interactions. Even when coformulation is possible, it may extend clinical development timelines. As a result, alternatives that allow administration of two separate injections while ensuring drug stability are being explored for both clinical development and commercial use.
CURRENT DELIVERY SOLUTIONS
Before injection, powder or lyophilised formulations must be reconstituted with a diluent, typically water for injection (WFI). This process requires careful handling and may increase the complexity for users, which may lead to higher risk of use errors. As self-injection becomes more common, there is a need for improved solutions that support non-HCPs in preparing and administering these formulations.
“THE SHIFT TOWARDS SELF-ADMINISTRATION OUTSIDE THE CLINIC TRANSFERS THE PREPARATION AND DELIVERY BURDEN FROM HCPs TO PATIENTS AND CAREGIVERS.”
Most lyophilised drug products in the market are provided in vial formats. In the Phillips Medisize internal review mentioned above, 67 of 71 approved products are presented in a vial or similar container, requiring users to add WFI into the drug container, mix and then withdraw the combined product for injection. While vial use is common in healthcare practice, the shift towards self-administration outside the clinic transfers the preparation and delivery burden from HCPs to patients and caregivers. Without professional training, the risk of use errors may increase, and the added complexity in preparation may affect adherence.
In competitive markets, such as immunology, the availability of alternative medicines offered in more convenient forms – for example, liquid-stable injections – may impact the uptake of new lyophilised formulations, even if these provide additional clinical benefits. When considering device preference, HCPs seem to take the number of steps involved in device use into account, which may influence their prescribing decisions.6
Given the complexities involved in preparing and administering lyophilised formulations, device technologies have been developed with the intention of reducing user burden and lowering the risk of use error. Table 1 summarises some of the solutions on the market that have been used to support patients administering injectable lyophilised formulations.
| Approach | Storage of Lyo Cake | Storage of Diluent | Delivery Device | Method of Use | Example |
| Two Vials | In a vial | In a vial | Separate syringe |
Syringe is used to draw diluent from first (diluent) vial and inject into the second (lyo) vial. The mixed drug is then withdrawn from the vial by a second syringe and is ready for injection. | OMNITROPE™ 5.8 mg/vial |
| Two Vials and Dual-Spike Transfer Device |
In a vial | In a vial | Separate syringe |
Diluent and lyo drug vials are connected using the transfer set. After mixing the final drug, product can be withdrawn via injection. |
Mix2Vial™ Transfer set (available as a device for use with multiple drugs) |
| Vial and Prefilled Syringe (PFS) | In a vial | In a PFS | Diluent PFS can be used to deliver the final product |
Diluent in PFS is injected into the vial containing the drug and mixed. After mixing the drug, product is withdrawn using a second syringe and is ready for injection. |
GATTEX™ (teduglutide) |
| PFS, Vial and Transfer Device, which has delivery needle pre-attached | In a vial | In a PFS | PFS integrated with the Mixject adapter | Prefilled diluent syringe is attached to the vial adapter. Diluent is injected into the vial via the adapter. After mixing the final drug, product is withdrawn from the vial into the diluent syringe. This is then detached from the vial and is ready for injection via the needle that was integrated into the transfer device. | BETASERON™ (interferon beta-1b) |
| Dual-Chamber Syringe | In the delivery device | In the delivery device | PFS used to store and prepare the injection | Drug and diluent are contained in a dual-chamber primary container. Partial movement of the plunger rod introduces the diluent into the lyo chamber via a bypass channel or valve. After mixing the final drug, product can be injected via a needle that is either attached or integrated with the syringe. |
GENOTROPIN MiniQuick™ |
| Dual-Chamber Cartridge (DCC) | In the DCC | In the DCC | DCC loaded into a delivery device such as a pen injector or autoinjector | Drug and diluent are prefilled in a dual-chamber syringe which is then loaded into the delivery device. Instructions to mix and deliver the drug are then device specific. |
GENOTROPIN™ Pen SKYTROFA™ Auto-Injector NEMLUVIO™ (nemolizumab-ilto) Pen |
Table 1: Commercial solutions that support patients administering injectable lyophilised formulations.
Unlike vials, in which the lyophilised drug and diluent are stored in separate containers, dual-chamber delivery systems house both components within a single primary container – divided into two chambers separated by a plunger stopper. The diluent is introduced into the chamber containing the lyophilised cake either through a valve, by piercing the stopper, or by moving the plunger stopper to open a bypass channel around it. From the user’s perspective, this approach simplifies the reconstitution process by reducing the chance of incorrect mixing, which is instead managed by the manufacturing process. Table 2 lists approved products that use a dual-chamber primary container.
| Product or Pipeline Name | Molecule/API Name | Formulation | Indication | Owner Company | Delivery Device |
| Systems Containing a Dual-Chamber Cartridge | |||||
| Atropine ComboPen Autoinjector | Atropine | Powder | Intoxication/Poisoning | MMT | ComboPen |
| Caverject™ | Alprostadil | Lyophilised Powder | Erectile Dysfunction |
Pfizer Inc | Caverject Pen Injector |
| DuoDote™ Pen | Atropine | Powder | Intoxication/Poisoning | MMT | Binaject |
| Edex™ Dual-chamber Cartridge | Alprostadil | Lyophilised Powder | Erectile Dysfunction |
Advanz Pharma | Reusable edex™ Injection Device |
| GENOTROPIN™ Cartridges | Somatropin | Lyophilised Powder | Growth Hormone (GH) Deficiency | Pfizer | GENOTROPIN Pen |
| GENOTROPIN GoQuick | Somatropin | Lyophilised Powder | GH Deficiency | Pfizer | Genotropin Disposable Pen |
| Natpara Lyophilized Powder* | Parathyroid hormone | Lyophilised Powder | Hypoparathyroidism | Shire/Takeda | Q-Cliq Reusable Injection Pen |
| NEMLUVIO™ Dual-Chamber Pen |
Nemolizumab-ilto | Lyophilised Powder | Atopic Dermatitis | Chugai | NEMLUVIO Dual-Chamber Pen |
| PEG Intron Redipen** | Peginterferon alfa-2b | Lyophilised Powder | Hepatitis | Merck and Co | BD Liquid-Dry Pen Injector |
| SKYTROFA™ Autoinjector | Lonapegsomatropin-tcgd | Lyophilised Powder | GH Deficiency | Ascendis | SKYTROFA Electromechanical Autoinjector |
| Systems Containing a Dual-Chamber Syringe | |||||
| Abilify Maintena™ | Aripiprazole | Lyophilised Powder | Schizophrenia/ Bipolar | Otsuka | Arte Dual-Chamber Prefillable Syringe |
| GENOTROPIN MiniQuick | Somatropin | Lyophilised Powder | GH Deficiency | Pfizer | MiniQuick DCS |
| Lupron Depot | Leuprolide acetate | Lyophilised Powder | Prostate Cancer | AbbVie | LuproLoc DCS |
| NEMLUVIO Prefilled DCS |
Nemolizumab-ilto | Lyophilised Powder | Atopic Dermatitis | Chugai | Vetter DCS |
| RenehaVis | Sodium hyaluronate | Powder | Osteoarthritis | MDT Intl | RenehaVis DCS |
| Suprecur | Buserelin acetate | Powder | Endometriosis | Sanofi | DCS |
| ViATIM Injection Suspension | ViATIM | Powder | Infectious Diseases | Sanofi Pasteur | ViATIM Dual-Chamber Syringe |
| XYNTHA™ Solofuse | Moroctocog alfa | Lyophilised Powder | Haemophilia A | Pfizer | SoloFuse Dual-Chamber Syringe |
Table 2: Approved products that use a dual-chamber primary container. *Discontinued globally 2024. **Discontinued in EU in 2021, in US in 2016.
Dual-chamber syringes offer a device option that supports both reconstitution and delivery from a single device. They can include either a staked needle or a needle attached via a luer lock connection, depending on the design. While dual-chamber cartridges (DCCs) do not include a needle, they can be incorporated into a device such as pen injectors or autoinjectors. Examples of devices incorporating dual-chamber primary cartridges include Pfizer’s GENOTROPIN™ pen (somatropin), Ascendis Pharma’s (Hellerup, Denmark) SKYTROFA™ (lonapegsomatropin-tcgd) autoinjector and Galderma’s (Zug, Switzerland) NEMLUVIO™ (nemolizumab-ilto) autoinjector.
Several studies have considered the usability and user preferences for different systems used to prepare and inject lyophilised drug formulations. Cimino et al7 evaluated five device scenarios for haemophilia A treatment. Four scenarios involved vial-based systems similar to those in Table 1, while one used a prefilled dual-chamber syringe (PFDS). The majority of the study was conducted as a survey with 299 participants who were experienced with haemophilia drugs. A subset of 98 participants also performed a simulated use study with the PFDS. Among this group, 57% preferred the PFDS over their current device, 26% preferred their current device and 17% found no preference. Overall, the survey data suggested a preference for the PFDS option.
In another study exploring the burden of at-home preparation of lyophilised injectable medications, Franzese et al asked 14 experienced participants to perform simulated use of one of four reconstitution methods: a double-ended spike adapter, a dual-chamber syringe, a prefilled diluent syringe or large-volume pooling (where contents of several lyophilised vials are combined and transferred into a cassette for infusion).8 Participants were distributed relatively evenly across the four approaches and performed a simulated use of the method relevant to their medication. The process was divided into three stages: assembly, reconstitution and transfer. Sessions were video recorded and analysed for deviations from protocol or potential breaches in sterility due to incorrect technique. A total of 85% of participants reported experiencing at least one preparation complication over the course of treatment with their current product.
In the simulated use study, seven out of eight instructions-for-use (excluding pooling) and all sterility breaches occurred during the reconstitution phase, indicating the importance of this step. The authors noted that all studied approaches imposed some level of burden on patients and emphasised the need for better, purpose-built reconstitution devices to help patients and caregivers prepare medication more efficiently and with steps.
The GENOTROPIN pen, NEMLUVIO autoinjector and SKYTROFA autoinjector aim to reduce patient burden and lower the risk of use errors compared with traditional vial-based and PFDS approaches. The NEMLUVIO autoinjector is a single-use disposable system delivering a fixed dose, the GENOTROPIN pen and SKYTROFA autoinjector are reusable devices supporting multiple drug presentations. SKYTROFA delivers fixed doses corresponding to the full cartridge content, whereas the GENOTROPIN pen supports variable dosing across multiple individual dual-chamber drug presentations.
Regarding patient feedback, to the best of the authors’ knowledge, there is a lack of published data directly comparing the usability of these more advanced devices with traditional lyophilised drug preparation methods previously described. However, a study on SKYTROFA involving experienced and injection-naive children and caregivers (N = 120), along with 15 HCPs, showed that all participants were able to successfully prepare and complete an injection using the autoinjector.9 The authors concluded that usability issues were low and comparable with results from other usability studies. All participants reported that they could follow the instructions as written, and 98% of participants felt that they could use the device as intended on their own or with supervision.
These examples illustrate some strategies that may be considered when selecting a device to support a dual-chamber lyophilised presentation. Increasing complexity – such as support for multiple presentations or variable dosing – may make reusable device solutions more suitable. Additional factors include safe reconstitution, where increased user burden might require greater attention to device usability support for critical user tasks.
“IN THE CONTEXT OF LYOPHILISED DUAL-CHAMBER DRUG PRESENTATIONS – WHERE INHERENT COMPLEXITIES EXIST – THERE IS POTENTIAL TO COMBINE USABILITY AND SUSTAINABILITY BY USING REUSABLE DELIVERY SYSTEMS.”
Environmental burden is a consideration influencing interest in reusable delivery devices. In the context of lyophilised dual-chamber drug presentations – where inherent complexities exist – there is potential to combine usability and sustainability by using reusable delivery systems.
The following section explores how the use of proven and pervasive technologies and, in particular, electronic technologies can support the development of improved delivery solutions for more complex and diverse programme needs.
APPLICATION OF ELECTROMECHANICAL DELIVERY TECHNOLOGY TO DUAL-CHAMBER DRUG PRESENTATIONS
Lyophilised medicines presented in dual-chamber primary containers may offer formulation and stability benefits but require reconstitution before injection. This additional preparation step, compared with using prefilled syringes (PFSs) or autoinjectors with a liquid-stable formulation, increases scope for risks in the overall administration procedure related to the user’s ability to correctly perform required manual operations during the preparation process. Examples of such risks include:
- Mixing WFI with the lyophilised cake before the needle is properly mounted could result in drug back-flush and a loss of dose due to pressure build-up in the drug chamber. Attempting to mix with the needle pointing downwards may also lead to unintended loss of dose.
- After the prescribed reconstitution period, the drug may require gentle swirling, repeated inversions or occasionally rigorous shaking to achieve sufficient homogeneity. The challenge is to ensure that users can replicate the process developed in the laboratory to support satisfactory mixing and reconstitution.
- The final step in the drug preparation process involves visual inspection to confirm the absence of particles or discolouration before injection. At this stage, priming may be performed by evacuating excess air from the front mixing chamber, while the user ensures the needle is pointing upwards.
In a previous article,10 it was suggested that the dominance of mechanical disposable injection devices may face competition from reusable devices, which offer potential advantages – such as greater user support and flexibility. These reusable devices can accommodate a wider range of formulations, including high-viscosity drugs,11 with minimal need for device modifications, unlike many spring-driven devices.
Given the complexities involved in preparing and delivering lyophilised drugs, electromechanical reusable autoinjector devices may offer a way to provide a user experience similar to that of a PFS autoinjector system.
In another article,12 Phillips Medisize explored how electromechanical autoinjector systems could assist in the preparation and injection of lyophilised drugs in DCCs. The SKYTROFA autoinjector serves as an example of this approach. Even without fully automating every process step, electromechanical autoinjectors can offer additional support to users, which may help reduce the likelihood of use errors. These devices may incorporate sensors that detect correct cartridge and needle mounting, as well as device orientation during manual handling. Combining these inputs with the device’s internal status – such as plunger position and timers – may allow the device to control the process sequence and communicate real-time guidance to the user. Graphical user interfaces, enhanced with animations and combined with focused acoustic and tactile signals, may further aid users throughout the process.
The development of electromechanical devices that can address some of the usability and patient burden challenges highlighted before provides a good example of how a company, like Phillips Medisize, with the right technical capabilities, can use its platform technologies in a flexible and versatile way to meet different sets of product requirements. This approach enables new developments, where possible, to use proven technologies from earlier developments to help reduce technical risk, time and cost.
For example, in the case of dual-chamber autoinjector development, subsystems such as the electromechanical drivetrain, electronic hardware and firmware, graphical user interface screen technology, sensors to monitor device orientation and passive needle shielding via a movable sleeve that also acts as a skin-contact can be used. This approach can lower technical risk compared with starting entirely new developments with untested technologies.
“USING PLATFORM TECHNOLOGIES MAY HELP ENABLE A MORE ROBUST SUPPLY CHAIN TO BE PUT IN PLACE AND DRIVE ECONOMIES OF SCALE IF COMPONENTS CAN BE USED ACROSS MULTIPLE PROGRAMMES.”
Moreover, the flexibility of electronics and electromechanical systems can allow changes to be made later in the development process with less impact on time and cost. The approach may also simplify reconfiguring the device technology for future drug programmes with different delivery requirements. Finally, using platform technologies may help enable a more robust supply chain to be put in place and drive economies of scale if components can be used across multiple programmes.
Although this article has focused on the complexities of preparing and delivering lyophilised formulations, DCCs can also be used to mix and deliver two liquids stored separately until the time of use. In many cases, the mixing process may be less complex. However, electromechanical delivery systems may still provide benefits in terms of usability and potentially support faster development timelines, particularly during clinical stages, and the use of electromechanical drive technology may make it easier to work with a wider range of viscosities for the two drug components.
CONCLUSIONS
Two-step mechanical autoinjectors for delivering liquid-stable drugs from PFSs have become a common approach for self-injection of biologic drugs, setting a benchmark for usability and patient convenience. While this approach generally balances usability, cost and device complexity, it does not address formulation challenges posed by some drugs that lack sufficient stability in aqueous solutions. Lyophilisation and the co-administration of separate drugs have long been options to address stability issues, but current device technologies face limitations in offering a user experience and use burden comparable with autoinjectors designed for liquid-stable formulations.
With emphasis on patient centricity and self-administration, the need to improve usability in preparing and delivering more complex formulations will only intensify. This article has highlighted how electromechanical delivery technologies have the potential to enhance the user experience in the preparation and delivery of lyophilised and liquid-liquid drugs from DCCs. Such technologies may also contribute to environmental stewardship (through device reusability), while offering user confidence by providing controlled processes and real-time feedback. Furthermore, the ability to incorporate technology that has already been proven on previous developments can facilitate a more modular approach to future developments, potentially reducing technical and timeline risks compared with a completely new approach starting from a “blank sheet of paper”.
Advances in delivery technology, combined with innovations in formulation and filling of dual-chamber containers, may expand options beyond traditional liquid-stable formulations. These developments may support accelerated time-to-market and enable delivery of new drugs that present stability challenges or require additional processing to remain stable in liquid form. By addressing these challenges and offering flexibility around different delivery needs, electromechanical delivery systems could play a role in shaping the future of self-administered drug therapies.
REFERENCES
- Martin KP et al, “Trends in industrialization of biotherapeutics: a survey of product characteristics of 89 antibody-based biotherapeutics”. Mabs, 2023, Vol 15, art 2191301.
- Uddin MN, Roni MA, “Challenges of storage and stability of mRNA-Based covid-19 vaccines”. Vaccines (Basel), 2021, Vol 9 (9), p 1033.
- Jiskoot W et al, “Ongoing challenges to develop high concentration monoclonal antibody-based formulations for subcutaneous administration: Quo Vadis?”. J Pharm Sci, 2022, Vol 111 (4), pp 861–867.
- Bhambhani A, Blue JT, “Lyophilization strategies for development of a high-concentration monoclonal antibody formulation: benefits and pitfalls”. American Pharmaceutical Review, Jan 2010.
- Nøhr-Nielsen A et al, “Body of evidence and approaches applied in the clinical development programme of fixed-dose combinations in the European Union from 2010 to 2016”. Br J Clin Pharmacol, 2019, Vol 85 (8), pp 1829–1840.
- Wood G, “A study of factors impacting user perceptions of disposable and reusable autoinjectors”. Presented at The PDA Miniverse: Medical Devices, Combination Products and Connected Health Conference 2025, Indianapolis, IN, US, Jun 2025.
- Cimino E et al, “Patient preference and ease of use for different coagulation factor VIII reconstitution device scenarios: a cross-sectional survey in five European countries”. Patient Prefer Adherence, 2014, Vol 8, pp 1713–1720. Erratum in: Patient Prefer Adherence, 2015, Vol 9, p 243.
- Franzese C et al, “The burden of at-home preparation of lyophilized parenteral medications: an analysis of contributing factors and implications for chronic disease patients and caregivers”. Expert Opin Drug Deliv, 2019, Vol 16 (3), pp 187–198.
- Lau M et al, “Patient-centric design of the lonapegsomatropin auto-injector for pediatric growth hormone deficiency”. Presented at the 25th European Congress of Endocrinology. Istanbul, Turkey. Endocrine Abstracts (2023) 90 EP745.
- Simpson I, “The new emerging needs driving autoinjector development”. ONdrugDelivery, Issue 113 (Oct 2020), pp 20–24.
- Simpson I. “High-dose drug delivery – how far can autoinjectors go?”. ONdrugDelivery, Issue 168 (Jan 2025), pp 90–94.
- Sørensen B, “Advantages of using an electronic injector for dual chamber cartridges”. ONdrugDelivery, Issue 67 (May 2016), pp 14-18.

