To Issue 138
Citation: “Interview: Arnaud Guillet, Vice-President Business Development, Biocorp”. ONdrugDelivery, Issue 138 (Oct 2022), pp 60–64.
Q Regular readers of ONdrugDelivery will be familiar with Biocorp but, for those who aren’t, please could you give an overview of the company?

Figure 1: Injay, Biocorp’s simple add-on connectivity solution for PFSs.
A Biocorp’s speciality is developing connectivity solutions that are useable across a range of drug delivery devices. For example, in the injectables space, our most famous product is the Mallya smart cap, version one of which is now on the market. With Mallya, we’re currently doing specialised developments with big players, such as Sanofi, Novo Nordisk and Merck KGaA. Another Biocorp product your readers may be familiar with is Injay, our smart prefilled syringe (PFS) solution, both for naked PFSs and PFSs equipped with safety systems (Figure 1).
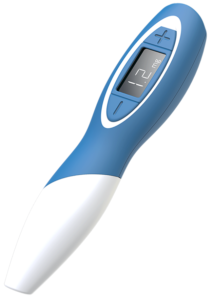
Figure 2: Datapen, Biocorp’s
reusable connected pen injector.
In fact, Biocorp was one of the first players to really investigate connectivity in the drug delivery space; we started the journey around 10 years ago. That’s how we launched the first connected device in the space – the Datapen (Figure 2), which is a motor-driven pen injector with Bluetooth Low Energy (BLE) connectivity. Datapen is still a platform that we are developing, although with more of a focus on the motor-driven aspect over the BLE connectivity part because markets are looking for very cost-effective solutions. Based on this experience, we quickly expanded into add-on solutions – there is a strong appetite for simple solutions. From the pharma and industrial standpoints, simplicity really is the critical factor. Seeing this, we quickly pivoted our development efforts to add-on solutions over fully integrated delivery devices, launching Mallya in 2014–15. And we’ve been developing the platform ever since.
Q Let’s broaden the scope – what’s your take on the current status of connectivity technologies in the delivery space as a whole?
A I would say that connectivity is currently most strongly influenced by a couple of internal and external factors. Starting with the internal factors, you’ve got device usability. When you’re looking at adding connectivity to a drug delivery device, it’s a complex problem – you’re adding electronics to an otherwise purely mechanical device, you’re adding extra functionality that needs testing and verification, you’re adding complexity for the patient who needs to use the device to deliver their medication. As you can imagine, switching from a very simple and purely mechanical device to an electronic device is anything but trivial.
“Another factor is the digital ecosystem, which adds significant
complexity because connected systems need to communicate
with each other.”
Another factor is the digital ecosystem, which adds significant complexity because connected systems need to communicate with each other. By default, they just don’t – if they’re not using the same application programming interface (API), they’re not speaking in the same language. Figuring out how to get connected devices to interface with everything they need to has been a very big challenge. Then, thinking more externally, you need to consider how you communicate with healthcare providers (HCPs). This iskey, because a huge part of the value of a connected device is its ability to deliver real device usage data to HCPs, who can then use it to engage further with their patients. A major consideration for anyone working in connected drug delivery devices is how to deliver the information gathered by the device to an HCP in a way that isn’t an additional burden for them. If HCPs see this technology as additional work for them, it’s never going to reach its full potential.
There is also the regulatory burden to consider. This is still a novel technology, and drug delivery is a risk-averse industry, so it has taken time and effort for regulators to understand connectivity and find where it fits in the regulatory model. When we first started to introduce connected products to regulators, we had to answer a lot of questions; “What are these devices?”, “Is an add-on considered part of the main device?”, “Are these combination products?”. In practice, there are three things to deal with for an add-on in this regard: the combination policy, the hardware and the software. Clear guidelines and regulatory pathways would be very helpful for seeing more successful developments in the connectivity space.
Naturally, this is all very complex by itself, but then you need to consider how it fits into the digital and pharmaceutical ecosystems. For example, you have data protection and data management to handle, which come with their own set of considerations, challenges and additional regulations. In tandem, there’s the question of how a connected product fits into the target market and, in particular, how it fits into that market’s reimbursement programmes. The lack of clear, value-based models for connected drug delivery devices has been an issue because adding connectivity creates an extra cost; where this leaves patients out of pocket, it acts as a major bias against adopting the technology.
Where we see some positive signs are where the biases imposed by these factors have been lifted. In this regard, covid-19 has had a notable influence here from a regulatory standpoint because, in the face of the pandemic, regulators understood that it was time to accelerate progress in digital health. We’ve seen them re-clarify the best way to proceed with these technologies and validate some applications on a provisional basis so that they can generate some real-world evidence and build their case. It’s really helped with reimbursement on certain applications, which is encouraging progress.
I also think that we’ve seen a positive trend in device usability. The miniaturisation of electronic components has enabled us to develop devices that are slimmer, closer to traditional delivery devices and have improved interoperability. Take the diabetes space as an example, it’s spectacular how you can now interface the data from your glucometers with mobile applications, artificial intelligence titration software and the data from a system like Mallya all together in a single place with a simple API interfacing tool. This development has been really helpful for driving up adoption.
“It ultimately all comes down to the same process – demonstrate the efficacy of the product, build your case, prove that you’re actually saving costs, boost patient engagement and show the value of the technology in the long run.”
Q How do you see different markets and healthcare systems factoring into the adoption of connectivity? For example, is there a difference between how the fully state-owned UK NHS and the US private insurance-based model have responded to connected drug delivery devices?
A That’s an interesting aspect to consider. In my view, while they are very different systems, both are well situated to adopt connected devices; the big difference is that it’s not going to be the same discussion. With the US, it’s going to be all about healthcare expenditure – the consideration is really based on the value and the cost effectiveness. Whereas, for the European markets, adoption will be much more driven by public opinion, so there we need to demonstrate what connectivity can do for patients.
That said, it ultimately all comes down to the same process – demonstrate the efficacy of the product, build your case, prove that you’re actually saving costs, boost patient engagement and show the value of the technology in the long run. You need to put money on the table, make sure you have sufficient time and collect sufficient statistically meaningful data. Having all of that in place will be key to seeing adoption in both markets.
Once everything is in place, it’s then a matter of who to have the key discussions with. In the US, it’ll likely be health plan providers, whereas it’ll be more state-driven in the UK, Germany and France. Germany in particular is an interesting case because of the Digital Health Apps (DiGA) Initiative, which will, based on limited evidence, clear an application on a provisional basis to give you some time to reimburse and prove that the product will actually yield some benefits for the healthcare system. It’s a good example of an original approach that could help foster connected technology.
Another factor in the US is the new current procedural terminology (CPT) codes for reimbursement and telemonitoring, which will enable us to engage HCPs more effectively about adopting connected devices because there will be a system for them to be paid for the time they spend on the software portal and dealing with the data fed back to them by the devices, rather than this simply being an additional burden.
In Europe, the way to significant adoption will really centre on building a body of clinical evidence. However, the European healthcare ecosystem is much more fragmented, so I think it will take longer to see widespread adoption, but it will ultimately reach a higher proportion of the population. There’s a cumulative aspect to it; every step we take forwards, the other areas can see success, which will make them more inclined to adopt connectivity themselves – it builds and snowballs as positive feedback.
Q Which therapeutic areas do you think are likely to lead the way in terms of adoption of connectivity?
A Diabetes is the stand-out case. It’s very complex – you have to use a number of data points from your glucometer. As a patient, you don’t necessarily know exactly how to interpret these data, so you need a doctor or an AI to interpret them and tell you what to do and how much to inject. This is an obvious case where digitalisation can greatly simplify the process and improve patient quality of life.
All this makes diabetes a good laboratory for connected devices, plus it’s an indicationwhere you can clearly demonstrate the impact on relapses and hospitalisations, which in turn shows the impact on the healthcare costs, society and patient wellbeing. Additionally, the adoption rate amongst diabetes patients is already quite high in terms of connectivity; I think part of that is a demographic factor – Type 1 diabetes patients are typically younger, and it’s Type 1 patients that are the trendsetters when it comes to connectivity.
Another category leading the way on connectivity is cardiovascular disease. Here, it’s not the utility of tools that will drive adoption, like with diabetes, but rather the risk associated with the disease. If a cardiovascular patient doesn’t stay in control of their disease, it can lead to very costly hospitalisations and serious healthcare complications. So, in the cardiovascular space, there’s real value in using connectivity to make sure that patients are managing their disease properly.
“The next logical step to tackle this non-adherence is to look into monitoring how patients are taking their prescribed medications and how they can improve patient adherence so that the drugs are as effective as under the clinical conditions.”
In a similar way, the respiratory sector is an interesting case. While it’s well known that poorly managed respiratory conditions, asthma and COPD in particular, are a massive burden on healthcare systems and life threatening for patients, it’s an enormous challenge to engage patients because the serious complications only come much later – sometimes patients don’t take their medicine for years before it happens. Connectivity is a potential solution to this challenge, so there’s a lot of potential in the sector.
Then there’s the developing market for biologics around indications such as multiple sclerosis, rheumatoid arthritis, Crohn’s disease and others that are delivered using PFSs and autoinjectors. This is an area we’re very interested in, because the biologics involved are very expensive and administered relatively infrequently, often only once every one or two weeks. Additionally, there is not necessarily a direct symptomatic incentive for patients to take them because they don’t experience a clear outcome from taking their medicine but, if they don’t, the consequences are severe. Therefore, I think that some payers will realise that, while they are reimbursing very expensive medicines based on the clinical results, in practice there are many hospitalisations as before. The next logical step to tackle this non-adherence is to look into monitoring how patients are taking their prescribed medications and how they can improve patient adherence so that the drugs are as effective as under the clinical conditions.
Q We’ve touched on interoperability and the digital ecosystem a few times up to this point, could you expand on Biocorp’s approach to this aspect of connected drug delivery?
A Absolutely. On its own, a connected device can monitor treatment adherence but not manage it. The treatment management part comes from the software and the service you’re accompanying it with, and there are some key considerations for how you implement it. How do you interpret the data that you receive? Do you use AI to process it automatically and expect patients to be autonomous or do you involve HCPs? How do you engage both parties? To succeed, it’s critical to map out the stakeholders and the patient journey.
The importance of thoroughly understanding the individual behind the patient is something we recently discovered. We want to invest more into behavioural science because, in many cases, while you’ll naturally need to tailor a digital solution to the target indication disease, patient non-adherence comes down to sociological, psychological or cultural issues that lead the patient to not take their medicine – it goes beyond just the complexity of understanding and using their device. This is why, on top of tailoring a solution for the disease, we need to provide answers for specific individuals to prevent non-adherence, so this is a key area of focus for us.
To this end, we want to invest in modern software that is not only able to give us full insight into the treatment, but also able to profile the individual user and fit them into different categories. So, when the software identifies a non-adherent patient, it will be able to determine that patient’s particular profile and tell what issue is relevant, and the application will tailor its response to make sure that the patient, with their specific needs, is addressed as an individual. For example, if you tend to forget your medicine, we’ll send you reminders; but if you don’t, and you don’t want to be bothered by them, we’ll address you in a different way. It’s really just tailoring the context and understanding the individual, so that we can engender sustainable engagement with the patient.
Q How is Biocorp gathering data and demonstrating the benefits of connectivity in terms of improving adherence and disease outcomes?
A Currently, we are building a lot of clinical trials for Mallya (Figure 3), as well as discussing and launching a lot of initiatives in this regard. For example, we are participating in a major European initiative called Trials@Home to prove the benefits of using Mallya together with connected glucometers and digital solutions to perform decentralised trials. The goal is to really demonstrate the feasibility, benefits and efficacy of these tools.

Figure 3: Mallya, Biocorp’s smart cap.
Beyond that, we are building some clinical studies to demonstrate the value of Mallya, both as part of the broader digital ecosystem, but also the intrinsic value of Mallya itself as just a smart cap. This is actually an excellent example of a keychallenge we’re facing across all connected device development – how do you isolate the contribution of a single device or piece of technology so that you can build a case for it?
Basically, there are two options. Either you build an entire digital ecosystem by yourself and build your case for the whole thing as a singular entity. This approach has some major drawbacks in that it’s not that flexible – you can’t integrate it with anyone else’s technology – and it’s very expensive. The second option is to find a way to demonstrate the benefits of your device as part of the much broader digital ecosystem. The challenge here is how do you design your clinical trial methodology? You need to find a way to isolate the value of your technology specifically, so you have to have a group that is just Mallya and another that is Mallya plus ecosystem and nothing on top of that. That way, you can examine the device factor in isolation.
It’s a real challenge. We’ve found some solutions to circumvent the issue but it’s an ongoing process. We’re planning to launch this initiative from next year, first at a European level to demonstrate proof of concept. Hopefully we can leverage the results of these trials in many European countries for further development, and also expand towards the rest of the world.
Q For Biocorp specifically, what’s coming next for the company over the coming months and into 2023?
A First and foremost, maturing our product portfolio will be an important aspect of the coming months. We have already launched Mallya V1 on the market, but there are also specific customised versions of Mallya for Sanofi and Novo Nordisk, so we’ll be maturing them and bringing them to market. As part of this, we’re looking to increase our manufacturing capabilities, including optimisation and automation, so that we are ready to meet any increased demand in a cost-effective manner. Accompanying that, we are also looking to increase our software development capacity, both so that we can boost our interoperability so that our devices can better interface with the other players in the market and also so that we can develop our own mobile app to work with our devices.

Figure 4: Sween, Biocorp’s injection assistant for patients with needle phobia.
On the sustainability front, we’re planning to conduct a major review to ensure that we are able to meet the demands of the market and potential partners. This is a fast-moving area, so it’s important to stay on top of it. For Biocorp, our products use plastic, they use electronics, so we need to optimise our sustainability profile. Fortunately, because we focus on add-on solutions, we tend to develop reusable solutions, which are naturally more environmentally sustainable. However, if we go disposable, it’s important that we do so in a manner with minimal environment impact, such as near-field communication (NFC) technology.
On the product side, we announced a new device at Pharmapak – Sween, an automatic needle insertion device to help patients who have needle phobia (Figure 4). We intend to bring this device to market on our own, targeting the end of 2023. We expect this device to see good traction because it’s tackling a critical issue and is in line with our traditional expertise in injection modelling and drug delivery submissions.

