To Issue 145
Citation: Koner J, Ershad A, Wyatt D, “Isothermal Dry Particle Coating – Back to the Future?”. ONdrugDelivery, Issue 145 (Apr 2023), pp 28–32.
Jasdip Koner, Abdul Ershad and David Wyatt introduce Aston Particle Technologies‘ novel dry powder processing technology – isothermal dry particle coating (iDPC™) – explaining how it has the capacity to enhance and broaden the scope of what dry powder formulations can achieve in respiratory medicine.
iDPC is a completely new way of processing powders at ambient temperature to create highly uniform mixtures without adulterating physical, chemical and biological properties. It is applicable to a wide range of particle types and can be used to coat smaller particles onto larger particles, utilising well established and controllable scientific principles.
FORMULATION FOR DPIs
Unit-dose dry powder inhalers (DPIs) have been in common use since the 1970s, key examples being Spinhaler (Fisons, acquired in 1995), Rotahaler (GSK), Breezhaler (Novartis) and HandiHaler (Boehringer Ingelheim). In the most basic form of a DPI, a drug capsule is manually loaded into the device, the device is primed, and the drug is drawn into the patient’s lungs by inhalation. The formulated dose contains minute quantities of micronised API combined with larger “carrier” particles, which are used as both a bulking agent and a dispersing aid.
“Device design has changed dramatically but the critical part of the inhaler – the formulation – has not.”
DPIs have evolved into more sophisticated multidose delivery devices over time, such as Turbohaler and Genuair (both AstraZeneca), Diskus and Ellipta (both GSK), Inhub (Mylan, now Viatris), NEXThaler (Chiesi) and RespiClick (Teva, Tel Aviv-Yafo, Israel). These evolutions have enhanced compliance, particularly in chronic therapy, improved drug delivery and become more patient friendly. Over this period, device design has changed dramatically but the critical part of the inhaler – the formulation – has not. This is because the technologies employed to produce homogeneous mixtures of micronised API with carrier particles have remained largely unchanged.
The current gold standards for formulation are high-shear blenders, originally designed for mixing liquids or slurries, and low-shear blenders, which, although designed ostensibly to mix powders, are highly inefficient when it comes to mixing cohesive materials. It could be argued that these blending standards have proved to be “good enough”, however, the truth is that the industry has learned to work within their limitations, including:
- The critical parameters of powder-mixing processes that depend upon shear are not well defined, so control strategies work with indirect measures rather than applied scientific principles. Such processes do not scale easily and the rule of thumb is that process development must be carried out at the largest scale available. This means that such work is typically highly inefficient.
- Fine powders are highly cohesive and at concentrations of greater than 5% w/w micronised API are extremely difficult to deagglomerate by mechanical shear of any kind.
- In high-shear blending, the formulation is exposed to excessive mechanical stress due to the impact of high-speed blades, impellers, choppers and baffles, resulting in local frictional heating and particle attrition.
- In low-shear mixing, tumbling energy is applied to mix powders and break down agglomerates; good content uniformity often requires multiple inefficient and wasteful sieving steps. In other cases, the powder is deliberately gently agglomerated by tumbling and the DPI device itself applies the energy to disperse the drug during the inhalation manoeuvre.
- Solid-solid interactions in the shear-blending process result in charge generation (triboelectrification) of the powder, which inhibits handling and requires the blends to be quarantined or conditioned before further processing. This extends the manufacturing time, and energy decay post-manufacture diminishes the performance of the product and limits shelf life.
The list could go on, so it is surprising that the industry has not made efforts to pursue better dispersion methods for dry powder formulation more vigorously. Good dispersability is particularly important for high-dose, low-potency compounds, as demonstrated by a small number of medicines such as Exubera (inhaled insulin, Pfizer), Tobi® Podhaler® (inhaled tobramycin, Novartis/Viatris) and Bronchitol® Orbital™ (mannitol, Pharmaxis, Sydney, Australia) that have come to market using smart engineered particles deliverable without a carrier.
While these are trailblazers for new formulations containing new components and novel physical forms, they introduce significantly more complexity into the drug development process and supply chains. There is a danger that such complexity will become the new standard. Undoubtedly, it is true that device innovation coupled with innovation in the manufacturing process is key to developing DPIs that are able to deliver better formulation performance. Aston Particle Technologies (APT) has an innovative new technology, isothermal dry particle coating (iDPC™), that may yet put traditional carrier-based formulations back on the map.
INTRODUCTION TO iDPC
iDPC was developed from research in the Department of Pharmaceutics at Aston University (Birmingham, UK). The aim of the project was to find a means of increasing the concentration of API in paediatric formulations by the direct coating of excipients onto the surface of the API particles. An extensive investigation of the existing dry powder coating technologies failed to identify any that could achieve the required degree of content uniformity, let alone the necessary surface coating.
By looking at the problem from scratch, an apparatus1 was constructed in which the particles to be coated, the “carrier”, are mixed with the coating particles, the “guest”, in a rotating chamber and dispersed together in such a way as to achieve both requirements. The process works through three simultaneous stages (Figure 1):
- De-agglomeration – The fast rotation speed/centrifugal force holds the powder at the wall of the vessel and distributes it in a thin layer. The centrifugal force also acts to pull apart any agglomerates of fine and/or coarse material into single particles.
- Dispersion – A nitrogen gas blade is injected and acts to disrupt the thin layer whilst also forming micro-pockets of fluidised powder in which the smaller, more mobile particles are readily dispersed throughout the coarse particles.
- Coating – within the micro-pockets of dispersed/fluidised powder the fine particles adhere to/coat the coarse particles.
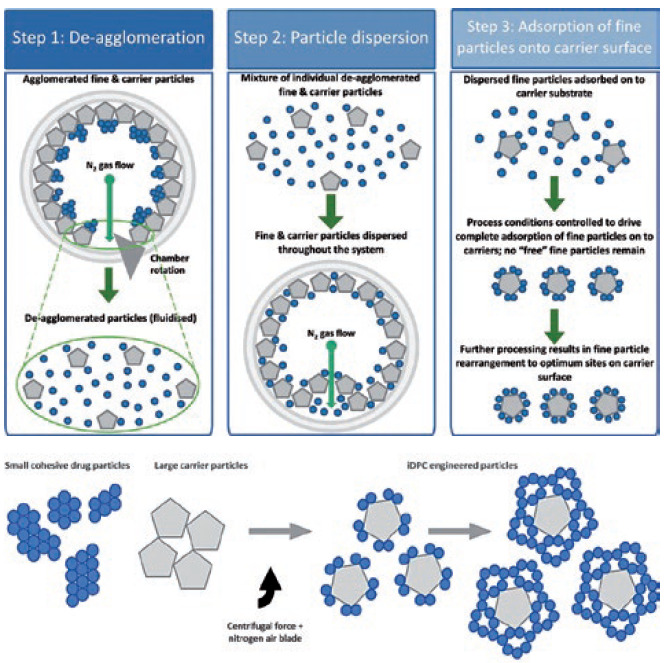
Figure 1: A schematic of the three steps within thin layer fluidisation.
These steps are distinctly different from the stochastic processes involved in conventional shear-blending techniques. There are no blades or baffles involved in the iDPC process, so highly energetic solid-solid interactions are avoided. In addition, there is minimal mechanical interaction with the particles, there are no dead spots, no frictional heating and reduced charge separation. The process runs at ambient temperature in an inert gas atmosphere and requires no temperature control or solvent use.
iDPC is a benign, exceptionally low-shear process because the bulk of the powder is held stationary during most of the processing, disrupted only by a gentle stream of dry nitrogen. iDPC is applicable to a broad range of large and small molecules – including biologics, which are not denatured by the process – and can be used to coat smaller particles onto larger particles, provided there is a size difference of 2–3 times.
iDPC FOR DPI FORMULATIONS
In iDPC, adjustment of the formulation composition can be used to create fractional coverage, monolayer coverage or multilayer structures on the surface of the carrier particles. This gives iDPC technology unique capability across the range of carrier-based formulation concentrations, from low doses with a single API to fixed-dose combinations (FDCs) such as those used in the treatment of asthma and COPD, and unprecedented high-efficiency delivery at high concentration, which could enable formulators to develop inhaled therapies for the direct treatment of lung infections, idiopathic pulmonary fibrosis and lung cancer, or even facilitate systemic treatment via the lungs. The following are examples that illustrate the capability of iDPC in the development of DPI formulations.
Exceptional Dispersion
Scanning electron micrographs (Figure 2) compare the structure of a formulation (A) and a sample from a commercial market-leading product of the same composition (B). In the iDPC formulation, the fine active is fully dispersed and coated evenly across the lactose carrier surface as single particles. The market leader, by comparison, contains agglomerates of fine particles deposited in a highly disordered manner across the lactose surface. This suggests that formulations manufactured by iDPC have undergone a more controlled coating process than that employed to manufacture the innovator product.
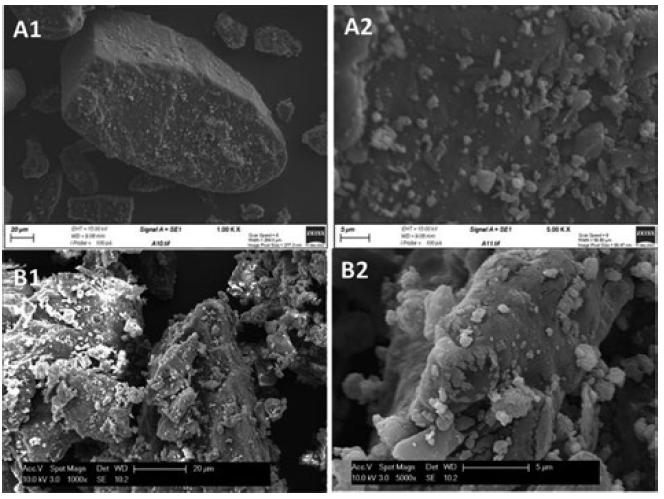
Figure 2: SEM images of an iDPC-processed formulation (A1, A2) and a commercial formulation with the same composition (B1, B2).
“iDPC is applicable to a broad range of large and small molecules – including biologics, which are not denatured by the process – and can be used to coat smaller particles onto larger particles, provided there is a size difference of 2–3 times.”
Exceptional Control
In iDPC, the blending of formulations is defined and controlled by the adjustment of just three critical process parameters – process speed, gas flow and process time – a science that is completely scalable. In contrast with conventional blending processes, which are notoriously difficult to optimise and scale, a quality-by-design approach is readily applicable to iDPC.
Figure 3 is a 2D response surface model from an experiment in which 30 formulations, including replicates, were prepared to explore the optimum operating conditions for the manufacture of a triple FDC formulation (salmeterol xinafoate/fluticasone propionate/glycopyrronium bromide). The extensive green areas show that the formulations deliver controlled and high fine particle fraction (FPF) performance for each active in the blend under a broad range of iDPC process parameters. This demonstrates that the manipulation of the iDPC process can deliver targeted, consistent and predictable DPI formulation performance. In manufacturing, a well-specified control space is a critical component of the chemistry manufacturing and control (CMC) section of any regulatory dossier, be it in the hands of an innovator company maximising performance or a generic developer targeting specific critical quality attributes to match innovator performance.
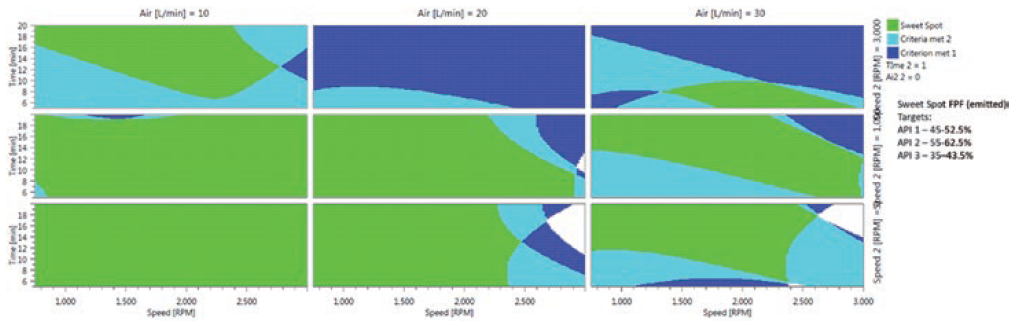
Figure 3: A 2D response surface model for a triple FDC formulation.
“iDPC has the potential to be the go-to platform for carrier-based DPI formulations, from controlled and predictable manufacture of traditional FDC formulations to the efficient delivery of high doses and anything in between.”
Exceptional Flexibility
The creation of high-performance, carrier-based DPI formulations with efficient aerosolisation, even at high concentrations, become possible with iDPC. Under traditional shear processing at high concentrations, cohesive binding of micronised particles drives agglomeration/aggregation, causing the efficiency of aerosolisation to diminish with increasing concentration. With iDPC at high concentrations, no such trend is found – the carrier particle surfaces are first saturated with fine particles and then further layers of API particles are cohesively bound. With iDPC, these multi-layered particles form a unique structure akin to a house of cards (Figure 4), a structure that is readily aerosolised to release individual particles. This is reflected in consistently high FPF across the concentration range from 5%–90% w/w API, a feat that cannot be replicated by existing high-dose DPI dispersion technologies (Table 1).
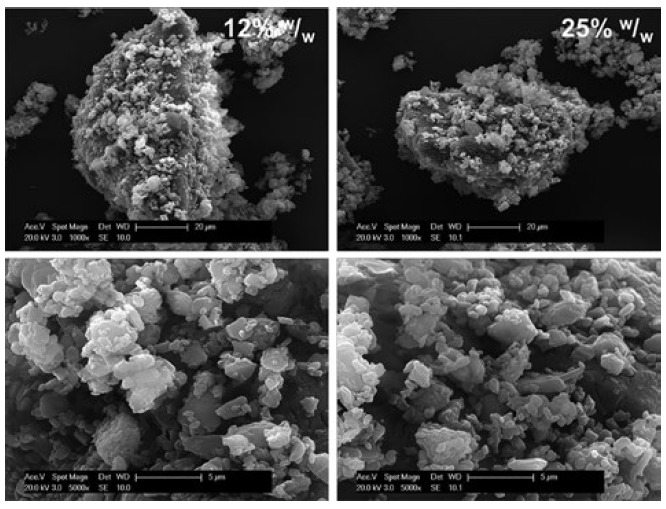
Figure 4: SEM images of an iDPC-processed lactose-based fluticasone propionate (FP) formulation with a multilayer structure.
| Concentration (% w/w) |
% Emitted | % FPF Emitted | Content Uniformity (% relative standard deviation) |
| 0.7 (control) | 92.2 | 27.2 | 2 |
| 5 | 72.7 | 50.3 | 1.5 |
| 12 | 77.8 | 56.8 | 2.3 |
| 25 | 61.1 | 45.6 | 1.8 |
| 50 | 65.1 | 56.6 | 2.7 |
| 75 | 72.3 | 51.8 | 2.3 |
| 90 | 79.7 | 51.2 | 1.2 |
Table 1: Aerosolisation performance of FP formulations produced using iDPC (evaluated using RS01, Qualicaps V-I capsules, next-generation impactor, 60 L/min).
THE POTENTIAL OF iDPC
The three attributes of iDPC described here are just scratching the surface of possibilities for its application. By taking a fundamental look at the principles of powder dispersion, the process of mixing cohesive, interacting particulate systems from “disorder to order” (ordered mixing2) can be exploited to a level that challenges the conceived wisdom in DPI formulation. Several advanced dispersion techniques, such as Mechanofusion (Hosokawa Micron) and resonance acoustic mixing (Resodyne, MT, US), are currently in use across the industry, however, APT believes that few have the promise of iDPC.
In an industry that is increasingly shifting towards lower potency molecules (small and large) for the treatments of respiratory diseases, and infection more broadly, iDPC has the potential to become a true one-stop shop for DPI formulation development. APT has a number of exciting programmes underway, one of which is to construct a plug-and-play system for high-dose, carrier-based DPI formulations. The object of this is to develop base formulations, suited to API types, properties and potencies, for rapid development into DPI formulations with highly efficient delivery – watch this space for updates.
FINAL REMARKS
This article has highlighted that iDPC has the potential to be the go-to platform for carrier-based DPI formulations, from controlled and predictable manufacture of traditional FDC formulations to the efficient delivery of high doses and anything in between. Not only does iDPC make formulation of DPIs more controllable, predictable and simpler; it makes DPI manufacture greener, leaner and more cost effective. APT is engaging and partnering with companies ambitious to develop the DPI formulations of the future.
Meet Aston Particle Technologies at RDD Europe (Antibes, France, May 2–5, 2023), where the company will be presenting scientific posters.
REFERENCES
- Mohammed A et al, “Coating Apparatus and Method”. Patent WO2016066462A1, May 2016.
- Hersey JA, “Ordered mixing: A new concept in powder mixing practice”. Powder Technol, 1975, Vol 11(1), pp 41–44.

