Citation: Arawaka S, Suzuki T “OXYCAPT Multilayer Plastic Vial and Syringe . ONdrugDelivery, Issue 113 (Oct 2020), pp 94–98.
Shota Arakawa and Tomohiro Suzuki discuss OXYCAPT™ vial and syringe primary containers, including the OXYCAPT™ material and product offering, as well as where Mitsubishi Gas Chemical sees OXYCAPT™ fitting into the modern pharmaceutical market.
INTRODUCTION
“The COP layers give OXYCAPT™ the traditional characteristic advantages of polymer syringes, while the new polyester plays a role as an oxygen and UV barrier to address the weaknesses inherent in using COP alone.”

Figure 1: The OXYCAPT™ multilayer plastic vial and syringe.
Although essential for humans, oxygen is basically unnecessary for processed foods and drugs. Over 40 years ago, Mitsubishi Gas Chemical (MGC) developed an oxygen absorber called AGELESS® which prevents the oxidation of foods. Since then, AGELESS® has been used in a variety of food products worldwide and MGC has been a leading company in the oxygen-absorber field. AGELESS® has also been used for drug products, such as intravenous (IV) solutions, prefilled syringes, ampoules and tablets, for many years, especially in the Japanese market. It significantly contributes to stabilising the efficacy of drugs and extending their shelf life. However, the use of an oxygen absorber is not as common in the US or Europe, as additional items, including dispensing machinery, sealing equipment and secondary packaging with high gas barrier, are needed to apply the absorber.
Therefore, MGC began developing alternative technologies to the oxygen absorber. Firstly, MGC developed a new oxygen-absorbing polymer, which featured a very low level of extractables and demonstrated no degradation, even after absorbing oxygen. Secondly, MGC sought an improvement on the existing multilayer-moulding technology which has often been used in the beverage industry to enhance the oxygen and carbon dioxide barrier provided by the packaging. By combining these two technologies, MGC has successfully developed a multi layered plastic vial and syringe called OXYCAPT™ (Figure 1).
OXYCAPT™ PRODUCT OVERVIEW
The OXYCAPT™ vial and syringe consists of three layers (Figure 2). The inner and outer layers are made of cyclo-olefin polymer (COP), the most reliable polymer used by the pharma industry. The middle layer is made of a novel polyester that has been developed by MGC. The COP layers give OXYCAPT™ the traditional characteristic advantages of polymer syringes (high water vapour barrier, very low extractables, high pH stability, low protein adsorption, high break resistance, etc.), while the new polyester plays a role as an oxygen and UV barrier to address the weaknesses inherent in using COP alone.

Figure 2: Multilayer structure of OXYCAPT™.
There are two types of OXYCAPT™ multilayer plastic vial and syringe – OXYCAPT-A and OXYCAPT-P. OXYCAPT-A has achieved a glass-like oxygen barrier. According to some internal studies, thanks to its oxygen-absorbing function, OXYCAPT-A can maintain lower oxygen concentrations in the headspace than Type 1 glass. OXYCAPT-P has also achieved an excellent oxygen barrier, although there is no oxygen-absorbing function. For example, the oxygen barrier of the OXYCAPT-P vial is about 20 times better than that of a COP monolayer vial (Figure 3). OXYCAPT-A is particularly suitable for oxygen-sensitive drugs and OXYCAPT-P is recommended for all drugs.

Figure 3: Oxygen permeability comparison of a typical COP, glass, OXYCAPT-A and OXYCAPT-P.
Barrier Properties
OXYCAPT™ is an excellent UV barrier. Although about 70% of UV light of 300 nm transmits through glass and COP, only 1.7% of UV light transmits through OXYCAPT™ (Figure 4). MGC has confirmed this feature also contributes to the stability of biologics.

Figure 4: UV light transmittance comparison of a typical COP, Type I glass and OXYCAPT™.
Regarding the water vapour barrier, OXYCAPT™ cannot reach the performance of glass. However, it is similar to COP, which has been used for injectable drugs for a long time, and easily meets the requirements of a water vapour barrier in International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines.
Extractables
Studies have shown extremely low extractables from OXYCAPT™. One study was conducted to confirm volatile, semi volatile and non-volatile impurities from OXYCAPT™. Water and four solutions (50% ethanol, NaCl, NaOH and H3PO4) were selected, and impurities were measured by gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-UV spectroscopy-mass spectrometry (LC-UVMS) after 70 days at 40°C. Compared with the blank, impurities were not detected in OXYCAPT™ containers. A second study confirmed that inorganic extractables levels from OXYCAPT™ were similar to those from COP, which is well known as an extremely pure polymer, and with a better extractables profile than Type 1 glass. Lower levels of inorganic extractables are known to contribute to better pH stability in drug products (Figure 5).

Figure 5: Inorganic extractables comparison of a typical COP, Type I glass and OXYCAPT™.
Syringe Construction
“MGC believes that OXYCAPT™ would be very suitable as a primary container material for epinephrine, because it is well known as an oxygen-sensitive drug.”
The OXYCAPT™ syringe consists of a tip cap, a barrel, a polytetrafluoroethylene laminated stopper and a plunger rod (Figure 6). Although a very small amount of silicone oil is sprayed on the stoppers of OXYCAPT™ syringes, no silicone oil is baked on the barrel. According to our internal studies using existing antibodies, MGC has found that this feature leads to much less protein aggregation compared with existing Type 1 glass syringes.
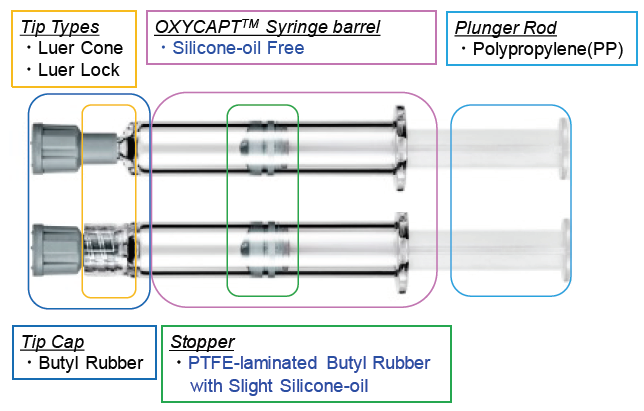
Figure 6: Components of an OXYCAPT™ syringe.
The OXYCAPT™ vial and syringe is produced by co-injection moulding technology. Although this technology has been applied to beverage bottles for many years, MGC is the first company that has succeeded in applying it to multilayer plastic syringes. MGC has also developed the inspection methods for the oxygen barrier layer. All the containers are 100% inspected by state-of-the-art machinery.
The latest dropping tests for these syringes were conducted based on ISO 11608-1:2014 (requirements and testing methods for needle-based injection systems). Gamma-sterilised OXYCAPT™ 1 mL long syringes and existing Type 1 glass syringes were dropped from a height of 1 m three times (once horizontally and twice vertically) onto a steel plate. Although 90% of the glass syringes were broken, no breakage was observed in the OXYCAPT™ syringes (Table 1).
| Samples | Numbers of Breakage at 1st Testing (for whole parts) |
Numbers of Breakage at 2nd Testing (for flange part) |
Numbers of Breakage at 3rd Testing (For lure part) |
Numbers of Syringes without Breakage through 3 Testing |
| OXYCAPT™ | 0/20 | 0/20 | 0/20 | 20/20 |
| Glass | 12/20 | 10/20 (From 1st testing: 5/8) (New: 5/12) |
2/20 (From 1st testing: 1/3) (From 2nd testing: 1/7) (New: 0/10) |
2/20 |
Table 1: Data from MGC’s latest drop testing of OXYCAPT™ and glass syringes.
OXYCAPT™ ON THE MARKET
MGC can offer bulk vial, ready-to-use (RTU) vial and RTU syringes. Regarding the RTU products, vials and syringes are provided in ISO-based nest and tub formats (Figure 7). The nest and tub are mainly sterilised by gamma ray. There are 2 mL, 6 mL, 10 mL and 20 mL for vials, and 1 mL long and 2.25 mL for syringes (Table 2). MGC is willing to provide samples for initial testing free of charge. Each polymer meets the requirements of USP 661, USP87, USP88 and EP, and has been filed in the US FDA’s drug master file (DMF). The vials and syringes are also compliant with each pharmacopoeia and have been filed in the DMF. The syringes are produced and controlled in accordance with ISO 13485.
| Type | Volume | ISO | Parts | Option |
| Vial | 2 mL | ISO 8362-1 | Vial | Bulk or RTU |
| 6 mL | ISO 8362-1 | Vial | Bulk or RTU | |
| 10 mL | ISO 8362-1 | Vial | Bulk or RTU | |
| 20 mL | ISO 8362-1 | Vial | Bulk or RTU | |
| Syringe | 1 mL Long | ISO 11040-6 | Barrel, Tip Cap, Stopper, Plunger Rod |
RTU |
| 2.25 mL | ISO 11040-6 | Barrel, Tip Cap, Stopper, Plunger Rod |
RTU |
Table 2: MGC’s OXYCAPT™ product portfolio.

Figure 7: Nest and tub storage for OXYCAPT™ vials and syringes.
The primary target market for OXCAPT™ is the therapeutic application of biologics. As mentioned in ICH Q5C (Stability of Biotechnological/Biological Products), oxidation is one of the causes of protein instability. As such, the oxygen and UV barrier properties of OXYCAPT™ will definitely contribute to the stability of biologics stored within. Also, some drug developers have started evaluating the OXYCAPT™ vial for their gene and cell therapy recently; the RTU vial is sterilised by gamma radiation, making it ideal for protein-based drugs.
In addition, MGC believes that OXYCAPT™ would be very suitable as a primary container material for epinephrine, because it is well known as an oxygen-sensitive drug. Storing epinephrine in glass also has the problem of glass breakage, a serious issue for an emergency drug, and therefore some suppliers have tried to develop new pen injectors using polymers as the primary container.
MGC has previously been asked to develop staked-needle multilayer plastic syringes by some of its customers. As such, MGC started tackling development a few years ago and recently decided to invest in a production facility for staked-needle syringes. The necessary equipment will be installed by March 2021. OXYCAPT™ syringe, with staked needle, has some special features, such as being free from tungsten, glue and adhesives, and will be available with several gauges and lengths (Figure 8).

Figure 8: OXYCAPT™ staked-needle syringe, currently under development.
CONCLUSION
In conclusion, OXYCAPT™ has been developed to solve the current problems in the pharmaceutical industry. In addition to the special features of COP, such as a strong water vapour barrier, high breakage resistance, very low extractables and low protein adsorption, OXYCAPT™ provides a strong oxygen and UV barrier. MGC believes that OXYCAPT™ brings a lot of benefits to the rapidly growing biologics and regenerative medicines market.

