Citation: Morel B, Authesserre C, “Safe and Automatic Device to Improve Drug Preparation from Multidose Vials”, ONdrugDelivery, Issue 120 (May 2021), pp 62–64.
Benjamin Morel and Claire Authesserre discuss the challenges and opportunities presented by multidose vials.
The use of multidose vials (MDVs) versus single-dose vials (SDVs) has been the subject of a 40-year debate. Yet the industry has been slow to implement alternative formats to SDVs, even though alternatives are being developed and can challenge existing practices.1,2
“EVEON’s technologies dedicated to automated drug preparation and injection demonstrate key advantages to overcome cost efficiency, healthcare worker usability and patient safety challenges.”
In 1983, it was already outlined by Sheth et al3 that MDVs could be used with relatively low risk of bacterial contamination. More recent studies demonstrate that using MDVs as packaging presentation for vaccines does not affect immunogenicity.4,5
However, this type of packaging is associated with some clinical outbreaks.6,7,8 The resulting manual handling can induce dosing mistakes and increase the risks of drug contamination and needle-stick injury. Therefore, MDVs require more training and more stringent practices to ensure patient safety.9,10
Furthermore, this type of packaging presentation has often been questioned from an economical point of view. The use of MDVs brings an additional challenge of increased dose wastage if opened with lack of planification during a vaccination campaign,9,11 and is inevitable, according to experts.12 In addition, studies have revealed that, during the H1N1 pandemic, MDVs were not as economic as expected due to the additional cost of time spent on manual handling and administrative processes.13
Today, more than 10 years after the H1N1 pandemic, MDVs are at the centre of the global strategic plan to overcome the current global crisis. Since the beginning of the covid-19 vaccination race, MDVs have been at the forefront. Worldwide demand for glass vials, as well as the glass technicity required for vaccine stability, and the low number of vials providers, led to the situation of potential shortage if only SDVs were used.12 To illustrate this point, a few weeks ago the US FDA approved two new MDVs, enabling a shift from six to 11-dose and 15-dose vials to overcome this situation.14 This measure is another step toward avoiding a vial shortage, but also a proven efficient measure to reduce what is considered “inevitable waste”.15
Indeed, the covid-19 crisis pushes forward the use of MDVs – but it is important to consider the beneficial aspect observed on the vaccination value chain and our ecosystem and to use them for more general purposes. We learnt from vaccination campaigns in low- and middle-income countries that MDVs enable a 40% packaging waste reduction16 and a 47% storage volume reduction in vaccination centres and storage rooms.16 Those benefits are key when you consider the waste management crisis, which has already started on a global scale.
That is why it would be of interest to see how to improve the use of MDVs and to challenge the current paradigm of SDVs. This global vaccination campaign is the right time to think out of the box, push innovations and overcome the drawbacks of MDVs. Identifying key learnings helps to further improve practices and bring new solutions for future local, regional or global vaccination campaigns.
One way to improve is to standardise the manual work performed by healthcare workers (HCWs) to get a repeatable, consistent, secure and cost-efficient vaccination process for each injection. This can be achieved through HCW training in best practice. Since manual work still remains user dependent, a medical device approach would enable the standardisation of preparation and injection steps by getting rid of inter-user variability and greatly limiting the number of manipulations.
One of EVEON’s goals is to address the challenge raised by drug preparation and injection. How can medical devices bring answers to questions raised around patient safety and cost efficiency? How can medical devices be part of the solution for reducing product wastage and improving usability for physicians?
EVEON’s technologies dedicated to automated drug preparation and injection demonstrate key advantages to overcome cost efficiency, HCW usability and patient safety challenges.
“An appropriate medical device, coupled with best-practice training, can fill the economic and patient-safety gap between MDCs and SDVs.”
By developing electromechanical devices, EVEON enables the automation of drug preparation and/or delivery. All parameters – such as the injected dose, the injection flow rate and the process time – are highly controlled, leading to a very reproducible preparation or delivery and a standard process, thus avoiding user-dependent variability, manipulation risks and dosing mistakes.
EVEON devices, using the company’s proprietary micropump, can deliver doses from 20 μL up to several millilitres with a high accuracy (<3%). The Intuity® Ject device has been used for several applications with different targeted injected doses. Table 1 shows the dose accuracy for four expelled doses. For example, a 1 mL bolus has been injected with ±0.01 mL, leading to 1% accuracy (n-6), and a 300 μL dose has been injected with ±5 μL, leading to <2% accuracy (n=30).
| Dose (mL) |
Expelled dose standard deviation (mL) | Dose accuracy (%) |
n |
| 3 | 0.1 | 3 | n=6 |
| 1 | 0.01 | 1 | n=6 |
| 0.3 | 0.005 | 2 | n=30 |
| 0.02 | 0.0005 | 3 | n=5 |
Table 1: Examples of doses expelled from EVEON devices for several targeted injected doses (3 mL, 1 mL, 300 μL and 20 μL) Errors bars correspond to standard deviations; n is the number of expelled doses used for standard deviation calculations.
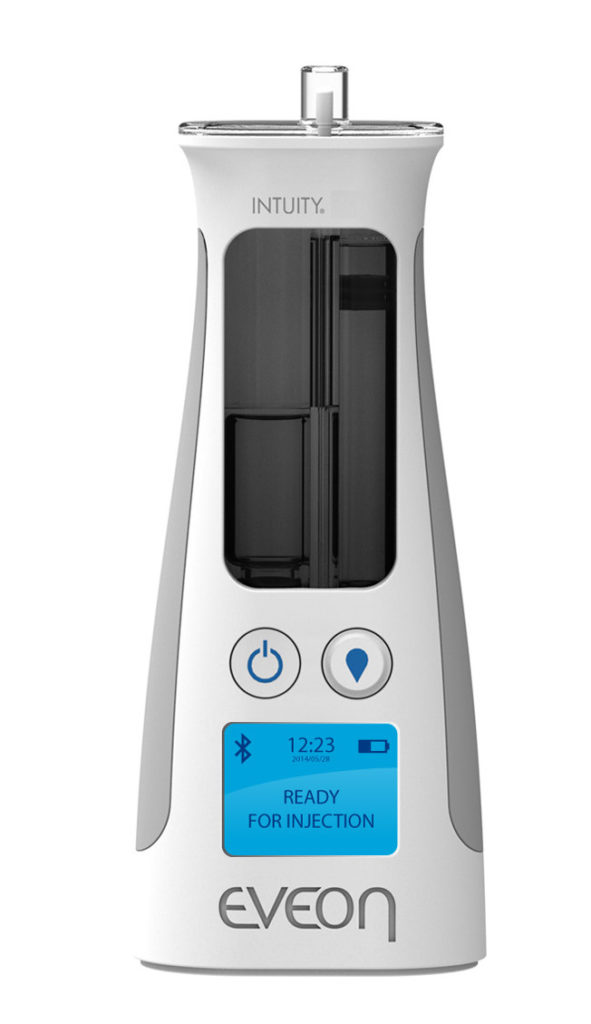
Figure 1: The Intuity® Ject device.
Such levels of dose accuracy with highly reproducible results allow optimisation of the filling of the drug container. In the case of MDVs, the extraction and delivery of an accurate dose allows for the same number of doses to be delivered every time, without requiring precise handling or losing time during the process. In the context of vaccine dose shortages, it also avoids the possibility of an HCW wasting a dose because of a wrong move.
The use of medical devices also enables control and reduction of dead volumes to maximise drug retrieval within the vial. EVEON devices are developed to be adapted to standard pharmaceutical containers. The design of the fluid path and the fluidic process is optimised to reduce dead volume and drug loss in both the containers and the device fluid path. EVEON’s development within its Intuity® Mix platform (Figure 1) demonstrates a 56–66% reduction in drug wastage within the vial, compared with other products on the market.
As HCW time is precious, especially in the context of a pandemic, EVEON devices are also designed to minimise the number of manipulations and the process time required for use. For example, the Intuity® Ject device (Figure 2) is capable of injecting a 300 μL dose in less than three seconds.
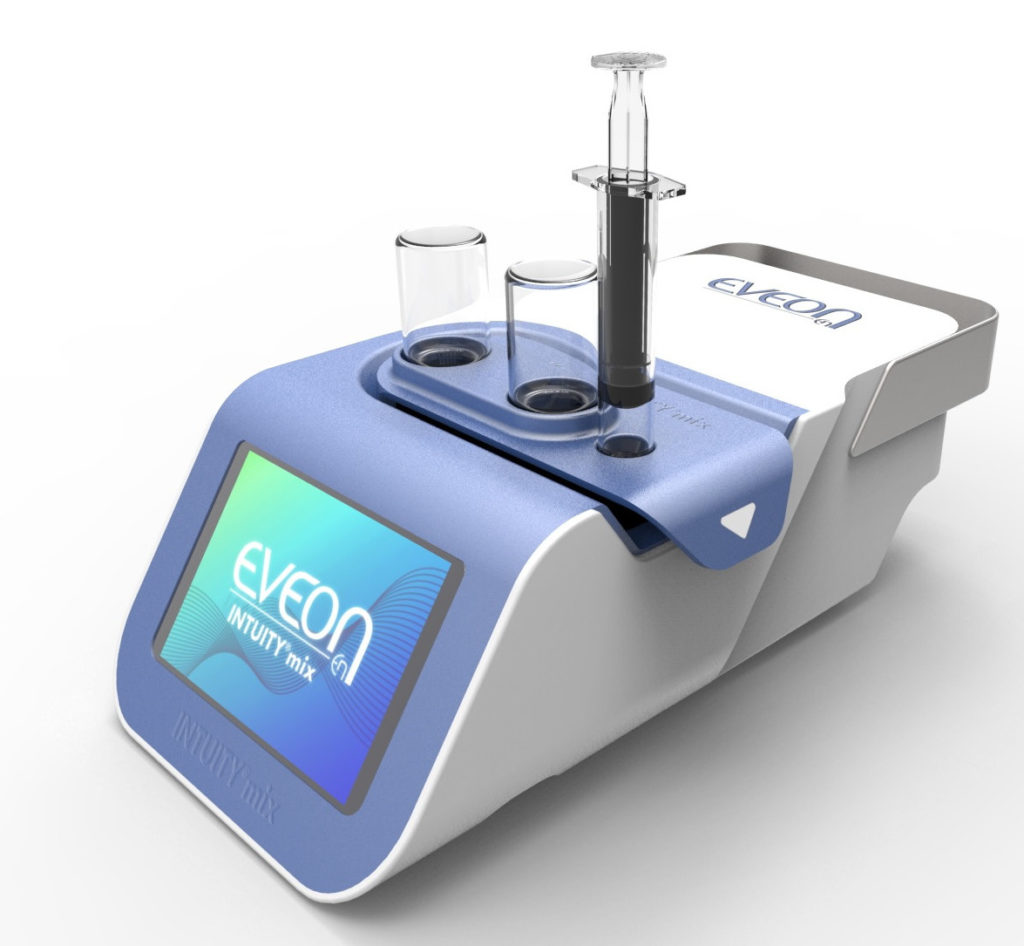
Figure 2: The Intuity® Mix platform.
Usability is also an important factor for time efficiency and device acceptability on the part of the user. That is why EVEON works in close collaboration with HCWs to understand users’ needs and the environment and use conditions of the device. EVEON is used to working with ergonomists and designers to take human factors into account, right from the first steps of device design, to be sure to design an appropriate and easy-to-use device.
An appropriate medical device, coupled with best-practice training, can fill the economic and patient-safety gap between MDVs and SDVs. EVEON can bring easy-to-use and robust medical innovation to standardise dose preparation (dilution) and injection – and turn MDVs into an attractive and competitive solution.
REFERENCES
- Sedita J et al, “Cost of goods sold and total cost of delivery for oral and parenteral vaccine packaging formats”. Vaccine, 2018, Vol 36(12), pp 1700–1709.
- Pagliusi S et al, “Vaccines, inspiring innovation in health”. Vaccine, 2018, Vol 36(48), pp 7430–7437.
- Sheth N et al, “Multidose vials versus signle-dose vials: a study in sterility and cost-effectiveness”. J Clin Microbiol, 1983, pp 377–379.
- Panda B et al, “Safety evaluation following immunization of pentavalent vaccine (multi-dose vial): experiences and comparative study”. Int J Contemp Pediatr, 2021, Vol 8(2), pp 243–247.
- “Vaccine wastage is one of the many challenges authorities balance during historic rollout”. ABC News, April 2, 2021.
- Miller D et al, “The safe use of multi-dose and single-dose vials”. SIS FactFinder, 2018.
- Chung Y-Seok et al, “A Large Healthcare-Associated Outbreak of Hepatitis C Virus Genotype 1a in a Clinic in Korea”. J Clinical Virol, 2018.
- Roseira CE et al, “Injectable medications: self-reported practices of nursing professionals”. Rev Esc Enferm USP, 2020, Vol 54.
- Kasi SG et al, “Prefilled syringes versus vials: Impact on vaccination efficiency and patient safety in Indian private market”. Pediatr Infect Dis J, 2013, Vol 5(4), pp 181–186.
- Tabatabaei SM et al, “A Study on Bacterial Contamination of Multidose Vaccine Vials in Southeast of Iran”. Int J Infect, 2017, Vol 4(4), e62887.
- Usuf E et al, “Vaccine wastage in The Gambia: a prospective observational study”. BMC Public Health 18, 2018, article number 864.
- Ojeda J et al, “Immunogenicity and safety of a multi-dose quadrivalent inactivated influenza vaccine in individuals aged 6 months to 17 years: a randomized phase III trial”. Hum Vaccin Immunother, 2020, Vol 16(6), pp 1380–1384.
- Pereira C et al, “Vaccine presentation in the USA : economics of prefilled syringes versus multidose vials for influenza vaccination”. Expert Rev Vaccines, 2010, Vol 9(11), pp 1343–1349.
- “Moderna COVID-19 Vaccine: New Multidose Vial; Updated Storage Conditions”. MPR, April 2, 2021.
- “The looming waste crisis that will follow COVID-19 vaccinations”. Devex, April 18, 2021.
- Kaucley L et al, “Decision making process, programmatic and logistic impact of the transition from a single-dose vial to a multi-dose vial of the 13-valent pneumococcal vaccine in Benin”. Vaccine, 2020, Vol 38(43), pp 6807–6813.

