Citation: Corrons-Bouis G, Le Loc’h C, “Spray Delivery: Targeting Patient Needs and Expectations with Precision”. ONdrugDelivery, Issue 154 (Nov 2023), pp 53–56.
Gladys Corrons-Bouis and Cyril Le Loc’h discuss the use of spray delivery for precise targeting of patient medication.
In recent years, new routes of administration have emerged and become more attractive for pharma companies. When one thinks of drug administration, one usually has in mind swallowing pills (oral administration) or getting an injection (parenteral administration). But drugs can be delivered through different routes, such as the enteral route through tubing in the gastrointestinal tract, inhalation through the mouth to reach the lungs or even a local route applied to the skin (cutaneous, transdermal) or to a mucosa (intranasal, buccal, auricular, ophthalmic).
Compared with conventional oral or parenteral methods, topical delivery brings key advantages:
- It is non-invasive and thus offers a convenient and patient-friendly delivery compared with injections – it offers a pain-free option for patients.
- It can avoid first-pass metabolism in the liver by allowing the drug to enter directly into the circulation system. This is an advantage when rapid drug absorption and onset of action are desired.
- It reduces the adverse effects induced by systemic drug delivery.
“Electromechanical spray medical devices … offer a controlled and efficient way to deliver high-value drugs.”
NASAL DELIVERY
Within local delivery, spray nasal delivery is particularly attractive – the rise of the nasal vaccine route is one example. Recent studies indicate that mucosal delivery of vaccines provides a better, long-lasting effect than the traditional injection route.1 Drug repurposing is also a big trend and an opportunity to innovate for pharmaceutical companies. Some older drugs have been repackaged as unit-dose nasal sprays. This allows a broader patient population to be targeted, increasing access to different end-user groups (for example, paediatrics).
Nasal sprays are also being explored as a non-invasive way to deliver medication to treat neurological conditions, such as epilepsy and certain psychiatric disorders, as they enable drugs to bypass the blood-brain barrier.
SKIN AND MUCOSAL DELIVERY
Pharmaceutical spraying technology is a simple method of delivering a drug onto the skin or to a specific mucosa and overcomes standard galenic form issues. The use of topical sprays as an application method for innovative wound therapies has been of interest in clinical practice for the past few decades, thanks to advantages such as the ability to cover large wound/skin areas, even in cases with unfavourable topography, reduced time for application and a homogeneous distribution of the sprayed suspensions.2
Sublingual spray delivery has gained attention in the last few years. It combines the advantage of sublingual delivery – bypassing the first-pass metabolism and providing a quick onset of action – and spray atomisation by distributing atomised droplets that enhance bio absorption.
OPTHALMIC DELIVERY
Spray delivery can also be a game changer for other administration routes. In May 2023, the US FDA approved MydCombi® – the first fixed-dose combination ophthalmic spray. Developed by Eyenovia (New York, US) and using the company’s Optejet microdose delivery platform, ophthalmic spray is a new alternative to eye drops.
Nowadays, most devices delivering doses in spray form consist of multidose dispensers containing a spring pump and a spray nozzle. Doses are delivered manually, and most of the devices contain preservatives that ultimately have side effects on the tissues. In addition, manual actuation of the delivered dose depends on the pressure applied to eject the dose.
As interest grows in spray delivery, companies need to develop innovations to improve the patient experience and ensure precise delivery. Electromechanical spray medical devices provide an alternative approach that addresses these challenges, offering a controlled and efficient way to deliver high-value drugs.
GETTING FULL CONTROL OF DELIVERY PARAMETERS
Customising a unique spray for each therapeutic treatment – or even for each patient – will help to ensure the right delivery of potent drugs. Allowing for precise control over the spray pattern, volume, dose, flow rate or angle is then key. As an example, self-administration is a challenge for many patients. Conventional spray systems are manually actuated and thus the spray dose and flow rate depend on the pressure applied to eject the spray.
Giving the option of adjustable dosing may allow patients to tailor their treatment. Using an electromechanical device, with embedded software controlling the motor and therefore the micropump, in combination with fine tuning the spray nozzle physical parameters, can have a direct impact on spray performance parameters, such as the surface area for administering the drug, the droplet size or the ejection speed. Electromechanical devices may guarantee total control of dose and flow, which can be adjusted according to requirements.
TARGETING HIGH PRECISION
Many innovative formulations can be fragile, making delivery a tough challenge. Delivering the right dose of a fully preserved formulation to the right area is essential. This is particularly crucial for medications with narrow therapeutic windows. Moreover, ensuring high precision of microdoses is key. Indeed, microdoses may increase local drug bioavailability and absorption, as well as avoiding adverse effects associated with systemic absorption.

Figure 1: The EVEON micropump.
EVEON micropump (Figure 1) technologies are a valuable solution to address this challenge. Specific micropumps have been developed for such applications, with the following specifications:
- Suction of a liquid from a cartridge and unidirectional delivery to a spray nozzle
- Electromechanical operation with small-size motorisation
- Pump displacement compatible with a spray dose of 15–30 μl
- Minimum pressure resistance of 10 bar
- Very short liquid ejection time of less than 100 ms with constant ejection speed
- Industrial feasibility for mass production in plastic injection moulding using medically compatible, sterilisable materials.
This new pump technology operates in such a way as to create high pressure for spray delivery. During the first suction phase, the pump is activated by a motor driving an oscillating-rotating movement of a piston, allowing a chamber to be filled and a spring to be compressed in the micropump. During the second delivery phase, the pump is activated by the spring release, moving the piston and emptying the pump chamber to eject the fluid through the nozzle.
The way the pump has been developed, in partnership with a plastic micro-injection-moulding specialist, and in parallel with the development of a very specific nozzle, makes it possible to administer a very precise dose in spray form in the shortest possible time and deliver fine, homogeneous and highly repeatable droplet mists.
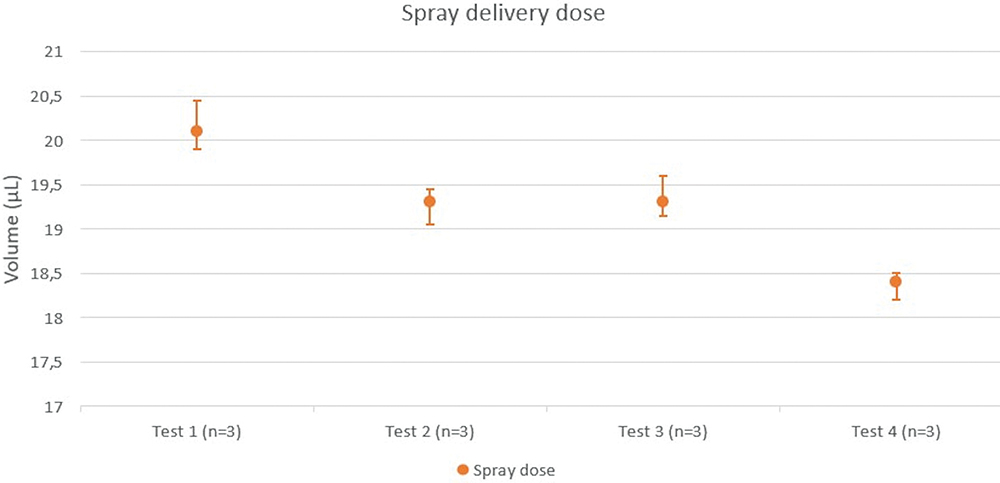
Figure 2: Test measures of spray delivery dose.
To characterise the spray dose delivered at the nozzle outlet, tests were carried out to precisely measure the volumes (Figure 2). The average dose ejected by the nozzle was 19.3 ± 0.4 μL.

Figure 3: The EVEON Intuity® Spray gives accurate airless spray or mist delivery.
The Intuity® Spray (Figure 3) integrates EVEON micropump technologies to create a spray device that will deliver precise and accurate microdoses.
“Intuity® Spray is a proprietary drug delivery platform designed for easy, accurate and precise dosing with a modern delivery device.”
NEW USER EXPERIENCE
Today, patients want new drug delivery methods that will simplify their day-to-day life. Healthcare professionals (HCPs) want to be sure that the right dose is delivered to the right area and that patient adherence is high. Connectivity features could enable HCPs to remotely monitor patient adherence and adjust treatment plans as necessary.
With connected devices, the embedded software and the associated features on the user interface can enhance the patient experience, monitoring by HCPs and thus treatment compliance. Such devices can, for example:
- Record delivery data, such as date and time of drug administration, number of doses delivered or remaining doses
- Integrate connectivity features (with a smartphone, for example) allowing data to be sent to the HCP
- Give information to the patient regarding battery level, dose remaining, treatment reminder, etc.
In an era driven by technological advancements, the spray device landscape is still dominated by conventional mechanical systems. Today, there is a need to shift to efficient and modern topical delivery systems. As an example, electromechanical device sprayers eliminate the need for manual pumping and standardise the delivery process. Automation facilitates the drug delivery procedure for the patient.
Although automation is key to ensure patient compliance, it is also important to gather user feedback as soon as possible in any device development to guide the design. EVEON carried out a formative evaluation (in the scope of IEC 62366-1:2015) on a panel of users representative of the target population for the application defined for Intuity® Spray platform. The study was based on mock-ups and guided the patient to follow the usage scenario with visual and audio feedback.
The aim of the study was to assess the following points:
- The shape and handling of the device, the way it is used, identification of the spray outlet, etc
- Overall understanding of the device’s functions and user interface, location of buttons, activation of the device and triggering of a dose
- Connection and disconnection between the consumable cassette and the reusable device
- Visual and audible signals.
User feedback was positive overall and led to a list of recommendations for the development of such a device:
Figure 4: Design for a better user experience.
- Immediate feedback
• The device must provide appropriate status feedback (visual or audible) in a short timeframe following the patient’s actions (pressing a button or connecting a cassette)
• The absence of feedback, or a long delay between the user’s actions and the feedback, can increase the risk of the user taking actions that interfere with the process. - Waiting times Long processing operations are indicated by a visual or audible signal with three orders of magnitude:
• 0.1 s – not perceptible to the user, no need to indicate this to the user
• 1 s – perceptible, an indicator is needed
• 10 s – the user can do something else, in which case the waiting time and progress must be announced. - Indicator lights
• Indicator lights are useful for communicating device status or for capturing the user’s attention
• On the other hand, several light indicators at the same time can be confusing, even in non-emergency situations
• Auditory indicators often work along with visual indicators (or replace them). - Colour indicators The number of colours used should be kept to a minimum. If possible, the device should be able to be used in “monochrome” mode. The following colour meanings are recommended:
• Green to indicate a normal, active state
• Red to indicate a faulty or abnormal state
• Orange to indicate a possible hazard
• White to indicate an undetermined state.
The next step involved implementing the user feedback recommendations into the design of the next generation of prototypes (Figure 4) with the goal of moving towards industrialisation and design verification and validation.
INTUITY® SPRAY
Intuity® Spray is a proprietary drug delivery platform designed for easy, accurate and precise dosing with a modern delivery device. It is a multidose and refillable device capable of delivering doses from 15–30 μL with 0.5 μL accuracy. The platform typically consists of:
- A reusable part that contains the electromechanical actuation and electronic controls
- A fluidic cassette – this interchangeable cassette holds the medication reservoir. The fluidic cassette contains:
– The medication, ensuring sterile and efficient delivery while allowing for easy replacement
– The micropump and the nozzle.
KEY FEATURES
- Microdose delivery: dose from 15 μL to maximum 30 μL in less than 100 ms
- Multidose delivery
- Topical administration of mist on a specific surface (Ø~10–12 mm)
- Droplet size mean of 20 μL with 90% of droplets under 100 μL and 10% under 10 μm
- Sterility preservation within the primary container/administered dose with an acceptable microbiological quality
- Portable reusable device, must fit in a pocket or handbag/must be easy to handle
- Primary container:
– Standard 3 mL cartridge currently developed
– Flexibility of existing containers for new developments - Connectivity – recording of delivery hours and dates, cartridge remaining doses count
- Rechargeable battery.
The Intuity® Spray platform uses an intellectual-property-protected architecture and is compatible with standard primary containers to minimise chemistry, manufacturing and controls development impacts for pharmaceutical companies and facilitate easy adoption.
CONCLUSION
Today, there is a clear need to shift to efficient and modern topical delivery systems. By tackling patient experience and dosing precision challenges, next-generation topical delivery devices are already starting to shift the industry.
REFERENCES
- Lavelle E, Ward R, “Mucosal vaccines — fortifying the frontiers”. Nat Rev Immunol, 2021, Vol 22(4), pp 236–250.
- Pleguezuelos-Beltrán P et al, “Advances in spray products for skin regeneration”. Bioact Mater, 2022, Vol 16, pp 187–203.

