To Issue 140
Citation: Jelgerhuis D, “Engineering Expertise: Design for Excellence”. ONdrugDelivery, Issue 140 (Nov 2022), pp 74–76.
Drew Jelgerhuis looks at how a design-for-excellence (DFX) systematic approach can help manufacturers achieve their objectives.
“The wide range of specialised fields of engineering makes it
nearly impossible to contain all the relevant knowledge that a subject matter expert can bring to bear to a design concept.”
STORY OF REGRET
Explosions, batteries overheating and burns were just some of the issues with a recent mobile phone product launch failure. According to Time magazine, this popular brand recalled 2.5 million of its new devices just weeks after it launched.1 Maybe your product launch has not been “burnt” in this way; however, not attending to design with all the factors associated with the product at the start could lead to similar failures. This major electronics company made mistakes in the product launch phase by not fully attending to design issues that became manufacturing issues both inside and outside the company.2 The reason for sharing this story of regret is to highlight the importance of design and using the design for excellence (DFX) systematic approach to achieve your objectives. This might include assembly, manufacturing, safety, cost and sustainability, to name those important to the market sector.
THESIS FOR DFX
Building DFX into your product development process provides a means for subject matter experts to offer valuable knowledge early enough in the design phase to reduce future risk, cost, time and failures. Many developers and designers in search of expertise will rely on specific design guidelines. Although much value can be derived from these guidelines, the wide range of specialised fields of engineering makes it nearly impossible to contain all the relevant knowledge that a subject matter expert can bring to bear to a design concept. During a recent design review with a customer focusing on multiple springs for an autoinjector platform, one of the developers said, “Springs are one of the hardest components to fully understand because of the depth of their technical complexity in a drug delivery device.” This applies in numerous categories of delivery system, including pulmonary (Figure 1), nasal, injectables (including wearables), and others, where springs play a crucial role, and is enough reason to engage expert spring designers early and throughout the development project using best-in-class simulation software.

Figure 1: Springs represent a crucial and yet complex component in inhalers and many other categories of drug delivery device.
WHAT IS DFX?
DFX is a systematic approach embedded in the product development cycle that requires a cross-functional team of experts to evaluate a product design focusing thoroughly and methodically on targeted objectives or characteristics. The chosen characteristics and objectives are represented by “X” and often focus on safety, cost, manufacturing and assembly. In recent years, sustainability has become a higher priority during the design phase, as well. A couple of specific “X” focuses are:
- Design for assembly (DFA) provides a method for simplifying the product design by minimising assembly operations and reducing components. The goal is to reduce assembly complexity, thereby improving throughput and reducing variation.
- Design for sustainability (DFS) focuses on material, processes, resources used and environmental impact, to name a few areas methodically evaluated and considered for changes to improve wholistically the sustainability of a product (Figure 2).
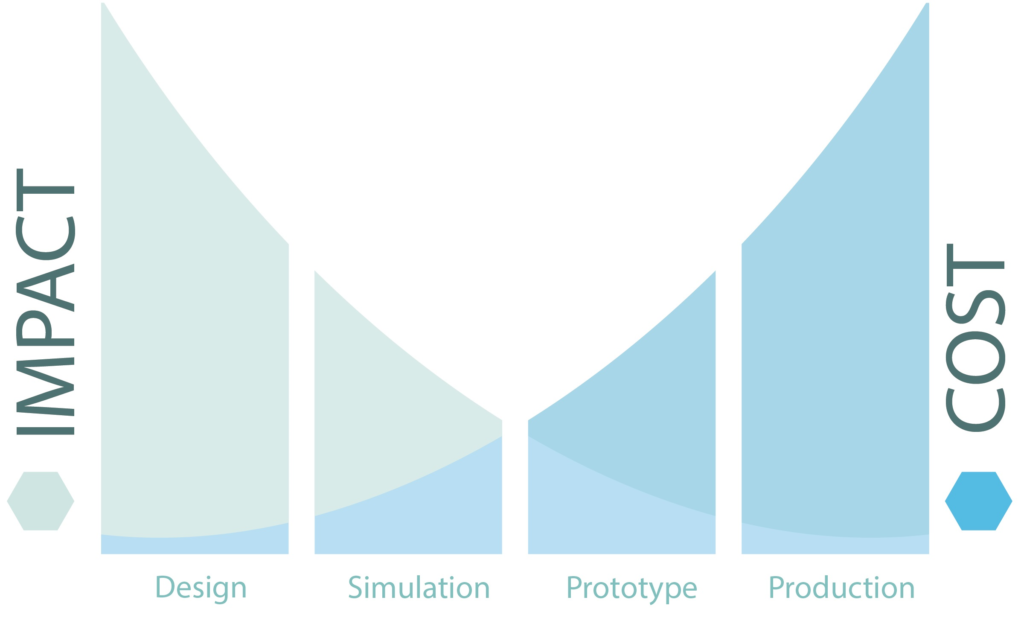
Figure 2: Impact cost diagram.
Scherdel Examples
Scherdel has been designing and manufacturing springs and precision stampings for over 130 years and has an extensive engineering team and technical resources. This depth and breadth of expertise that Scherdel can provide early in the development phase will reap great benefits in the later phases of launch of the product. Some examples with recent customer developments include automated assembly, part-design input to minimise quality issues, patient requirement characteristics affected by part-process tolerances and part-handling considerations for high-volume production. Using DFA as the focus, here are four relevant examples.
The first example is for a current customer that is producing low-volume product manually and is ramping up production with projections for significant volume increase in the coming years. Due to Scherdel’s experience in both high-volume spring production and automation equipment, the company worked closely with this customer on various concepts for automation that would significantly reduce labour, improve quality and reduce material cost with minor design changes. After several iterations, the companies were able to agree on a concept that reduced labour by more than 75%, paid off the automation in under 18 months and provided upwards capacity from the current 100,000 parts per year to over 1.2 million per year.
Another example involved redesigning two parts to be identical and symmetrical about a central axis. This allowed the part to be bowl fed in one bowl instead of two and picked up by the same robot gripper, and eliminated a costly and timely vision system that would have been necessary to identify the two different part designs. Had this DFA been conducted before the part was designed and tooled for injection moulding, it would have saved the company around US$30,000 (£26,500) in tooling cost and $15,000 per year in labour.
A third assembly related example involves working with a start-up in the drug delivery business. This customer is designing a novel delivery device that requires multiple springs. Scherdel conducted multiple design review sessions focusing on the specified force to meet the delivery needs of the drug in various viscosities while still meeting patient safety and comfort characteristics. The design was optimised by designing both right- and left-hand wound springs to balance the torque load during deployment of the drug. By designing the ends of the springs and the corresponding component they nest in with error proofing features, the parts could not be assembled incorrectly and yet can be fed with automation and no tangling issues often associated with springs that are fed automatically.
A final assembly example involves a combination device that required several different springs. Scherdel’s design engineer engaged in many design review sessions focusing on eliminating grinding, tangling and tight tolerance issues. The grinding that was initially required was eliminated by suggesting square wire in place of round and a redesign of the mating component. The tangling issue was resolved by including an innovative spring redesign that did not impact the component characteristics. The tolerancing concern would have required a slower process and higher-cost in-line inspection but, by changing the design, the tolerance could be opened and not affect the very critical free length specification.
BENEFITS OF DFX
As evidenced by the examples, the cost-benefit ratio is much greater the earlier Scherdel’s design team can engage with the product development team. This involvement early on with subject matter experts, such as design engineers, can arguably reap, as an MIT research paper suggests, 50%–65% savings.3 Certainly, the company has specific examples where savings of the spring or stamping component was reduced by at least this amount when redesigned. In addition to the assembly examples shared, Scherdel can also bring its subject matter expertise to bear on safety, material and sustainability topics related to product design (Figure 3).

Figure 3: Scherdel’s design team provides inputs supporting concepts, simulation, prototypes, testing, tooling and automation, all based on experience gained from serial production of hundreds of millions of drug delivery springs and stampings.
Scherdel provides best-in-class design input in the form of concepts, simulation, design, prototypes, testing, tooling, automation and, of course, serial production of hundreds of millions of drug delivery springs and stampings. Scherdel leverages its team to drive significant cost out of your product while improving quality, safety and environmental impact.
REFERENCES
- Samuelson K, “A Brief History of Samsung’s Troubled Galaxy Note 7 Smartphone”. TIME, Oct 11, 2016.
- Heathman A, “We finally know why Samsung’s Galaxy Note 7s ‘exploded’”. Wired, Jan 24, 2017.
- Whitney DE, “Manufacturing by Design”. Harvard Business Review Magazine, Jul 1988.

