To Issue 156
Citation: Berardi L, “Stainless Steel Needles: Quantifying Cobalt Risk to Comply with EU Medical Device Regulation”. ONdrugDelivery, Issue 156 (Jan 2024), pp 68–72.
Laura Berardi discusses the reclassification of cobalt as a Category 1B carcinogen and presents a series of analytical studies into the toxicological risk present in needles and complete syringes.
From digitally enabled autoinjectors sophisticated syringes, all medical products and devices are designed around the same clear and simple objective of improving patient wellbeing. Their success is dependent on the integration of a variety of component parts, all of which must contribute positively to the whole.
If these strict parameters are not satisfied, however, there is potential for a paradoxical situation to arise where a medical device or component, despite being designed to benefit a patient, becomes a source of discomfort or physical harm. At the most extreme end of the spectrum, the potential exists for a device to unknowingly introduce unwanted compounds or elements into a patient’s system, contributing to the development of life-limiting illnesses.
“Cobalt has been shown to have serious harmful effects on the lungs, triggering allergies and skin reactions as well as having a carcinogenic effect.”
An example of such an element is cobalt. A naturally occurring trace metal, cobalt is present in the environment and is employed in a variety of commercial applications, from rechargeable batteries to agricultural fertilisers. While it can be ingested through respiration or via the skin, cobalt typically enters the human body through the consumption of food or vitamins, and at normal blood plasma concentration levels of less than 0.2 μg/L it is beneficial as a contributor to the production and regulation of red blood cells, platelets and DNA, while also supporting the synthesis of fatty acids and energy production.1,2
At levels above this, however, cobalt has been identified as a potentially serious patient risk. Cobalt has been shown to have serious harmful effects on the lungs, triggering allergies and skin reactions as well as having a carcinogenic effect.
Indeed, in 2017, the Risk Assessment Committee of the European Chemicals Agency proposed reclassifying cobalt as a Category 1B carcinogen based on evidence from studies that found that exposure at toxic levels resulted in an increased incidence of lung cancers.
Furthermore, the International Agency for Research on Cancer conducted an evaluation of the carcinogenicity of cobalt and other metals, which resulted in cobalt metal and soluble cobalt(II) salts being designated as “agents that are probable carcinogens to humans”.3
The reclassification and reassessment of cobalt, while not directly driven by concerns relating to medical devices, has had knock-on effects for the pharmaceutical sector. Cobalt’s properties, such as biocompatibility, corrosion resistance, strength and durability, mean that it can be incorporated into certain materials and components used in medical devices at times. It can also be present as an unintentional impurity, with small amounts sometimes present within stainless steel grades and, therefore, ending up in products such as needles.
Theoretically, this situation could present a risk for patients who either receive or self-administer injections on a frequent basis. In such cases, the repeated contact with cobalt-containing needles has the potential to result in toxic levels of cobalt migrating from the material and into the patient.
Part 3 of Annex VI to the CLP Regulation (EC) No 1272/2008, also the European Medical Device Regulation (MDR), dictates that medical devices containing >0.1% w/w of substances that are Category 1A or 1B carcinogenic, mutagenic or toxic to reproduction (CMR) must incorporate appropriate labelling and a justification for the use of that substance. This is set out within Annex I of the MDR in paragraphs 10.4.1 (Design and manufacture of devices) and 10.4.5 (Labelling), which refer to substances classified as CMR for integral medicinal products and medicinal products with co-packaged devices.4
“The repeated contact with cobalt-containing needles has the potential to result in toxic levels of cobalt migrating from the material and into the patient.”
While the MDR provides a clear threshold for accepted levels of CMR substances, it does not provide clear parameters for how these levels can be determined. More specifically, the MDR does not outline whether the CMR content and concentration levels should be measured at a component level or in the context of the entire product, following the completion of all manufacturing and assembly processes and after being exposed to storage conditions. For pharmaceutical companies following this guidance and seeking to guarantee patient safety, this raises important questions over how the MDR should be properly interpreted and, at a practical level, whether analysis of individual product components provides a sufficient basis from which to confirm compliance in relation to safe CMR content and concentration levels.
Stevanato Group, as a market-leading supplier of drug containment solutions and drug delivery systems, sought to provide clarity on these questions by conducting a series of analytical studies into the potential toxicological risk present in needles and complete syringes. As well as reinforcing the company’s technical capabilities in analytical services, this project also underlines Stevanato Group’s commitment to providing pharma partners with the highest level of support in the supply of patient-safe drug delivery components and devices, ensuring that risks are fully managed within complex production environments and throughout multi stakeholder supply chains.
A chemical characterisation study was designed to determine extractable amounts of chemical compounds present at a product and component level, allowing the toxicological risk to be verified. The first part involved extraction analysis of complete 1 mL EZ-fill® syringes assembled with stainless steel needles (AISI 304) from three different suppliers and separate extraction analysis carried out on needle components from three different suppliers that were not incorporated into a final assembled product.
The 1 mL EZ-fill® syringes, having undergone double ethylene oxide sterilisation, were extracted with ultrapure water (UPW) at 121°C for 60 minutes and with hexane at 58°C for 24 hours. The extracts were then subject to a wide range of analytical methods ensuring detection and identification of glue-based organic compounds, elements and anions.
Separately, the isolated needles were exposed to three increasingly aggressive conditions to generate an extract: UPW; UPW pH2 (± 0.5); and UPW pH9 (± 0.5). This analysis was conducted using closed-vessel shaking incubation (~50 min-1) at 50°C for 72 hours.
In the second part of the study, the surface of the needles was closely analysed, both in isolation and as part of an assembled syringe. This analysis was conducted using scanning electron microscopy – energy dispersive spectroscopy (SEM-EDS), which generates a profile of the compounds on the surface of the samples and information on the morphology of the needles. Specifically, the analysis was performed using a ZEISS Sigma field emission scanning electron microscope (FE-SEM) with 1.5 nm of maximum resolution.
The technique involves using a focused electron beam as a probe to inspect the surface of the solid material, with a focus on the needle bevel. Different integrated detectors can be used to collect the generated particles, allowing for evaluation of the sample’s morphology. EDS analysis was performed on the same area as the SEM inspection with a Quantax Bruker detector to characterise the elemental composition of the stainless steel.
In the extraction analysis, the results of the needle samples tested with UPW, UPW pH2 and UPW pH9 provided a measure of the amount of various extractable compounds in each test article unit. In the case of UPW, total organic carbon (TOC) was identified in the highest amount, ranging between 0.04 and 0.08 μg/unit. The next highest compounds recorded were calcium (0.03 μg/unit), nickel (0.025 μg/unit) and silicon (0.026 μg/unit).
In the case of the UPW pH2 extraction, the compound recorded at the highest level was iron, with an outlying peak result of 1.89 μg/unit for one of the sample needles. All other compounds were recorded at levels of 0.1 μg/unit or below, with many measured at negligible levels. In the case of the UPW pH9 extraction, ¬TOC and silicon were the only compounds to be recorded at a level of 0.03 μg/unit or above. Crucially, for all samples across all extraction tests, the presence of cobalt was not detected in any needle (Figure 1).
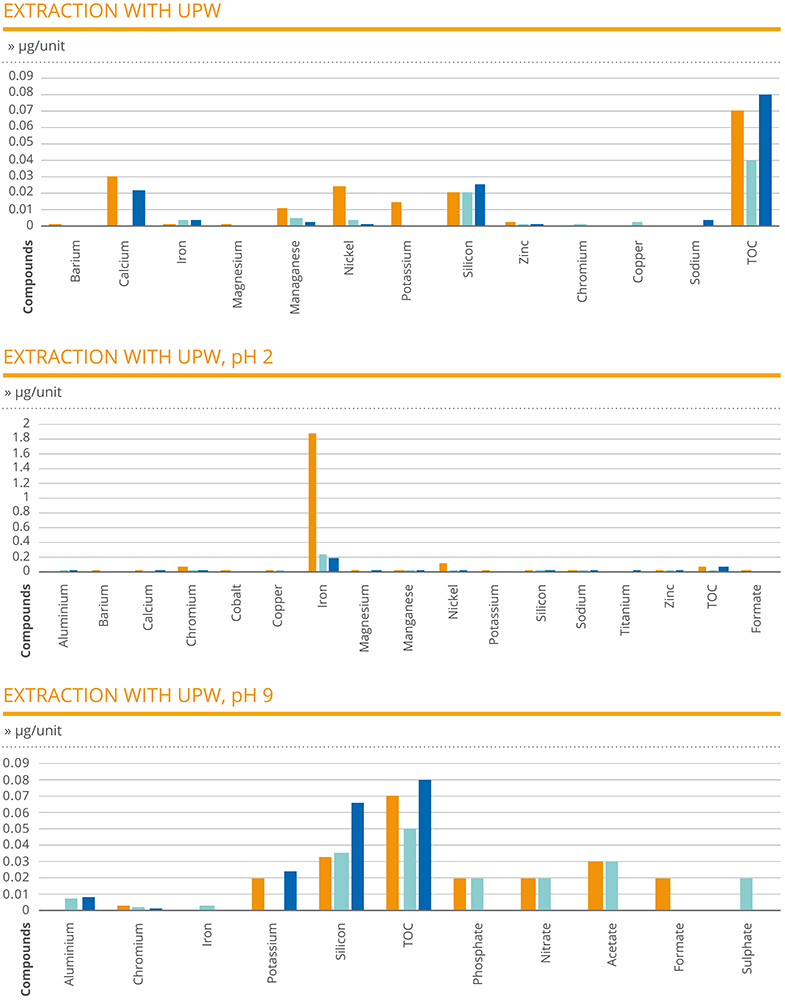
Figure 1: A chemical characterisation study was designed to determine extractable amounts of chemical compounds present at a product and component level, allowing the toxicological risk to be verified.
The extraction analysis carried out on the 1 mL EZ-fill® syringes revealed silicon as the compound at the highest level, ranging between 0.018 and 0.055 μg/unit. Apart from sodium, which had a range of 0.012–0.018 μg/unit, all other compounds were found to be at negligible levels. Some compounds were only detected on certain samples: sulphur was only found on syringe units employing two of the needle types, for example, while tungsten and zinc were found on syringes that employed the third needle type (Figure 2).
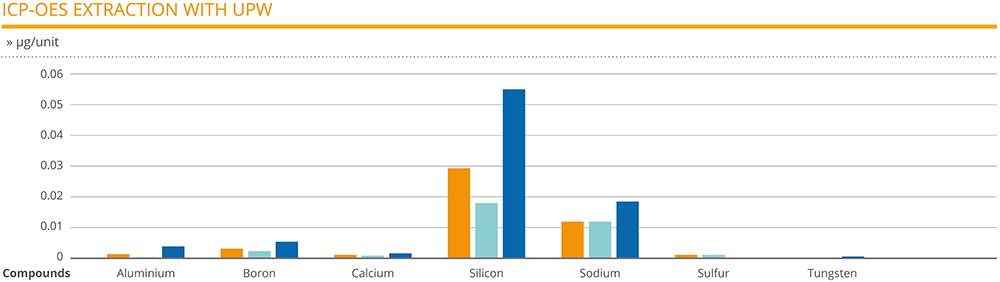
Figure 2: 1 mL EZ-fill® syringes with three different needles and double ethylene oxide sterilisation.
“In the face of potential uncertainty over the presence and concentration of a substance such as cobalt, which is classified as CMR, these findings provide unequivocal certainty that levels are within compliant levels at a product and component level.”
The results from the second part of the study, which analysed the surface of the needles as both standalone components and assembled within EZ-fill® syringes, found no morphological differences between the component needle samples and the assembled syringe samples. The spectrum profile generated by the energy dispersive spectroscopy pointed to higher quantities of silicon, oxygen and carbon on the assembled needles as a result of the manufacturing process, but it did not highlight the presence of cobalt (Figure 3).
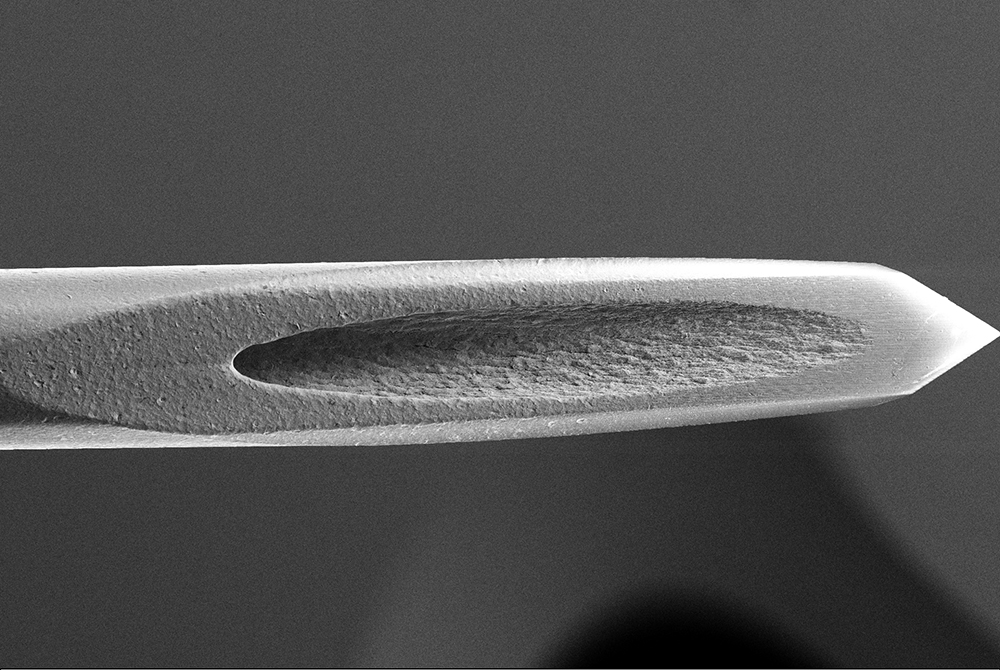
Figure 3: The spectrum profile generated by the energy dispersive spectroscopy pointed to higher quantities of silicon, oxygen and carbon on the assembled needles as a result of the manufacturing process, but it did not highlight the presence of cobalt.
Taken together, the detailed data obtained from both parts of this extensive study conducted by Stevanato Group provides a clear picture of the chemical compounds that are both present on and contained within assembled syringe products and individual needle components. The insight derived is invaluable for pharmaceutical companies developing medical products compliant with the MDR.
In the face of potential uncertainty over the presence and concentration of a substance such as cobalt, which is classified as CMR, these findings provide unequivocal certainty that levels are within compliant levels at a product and component level. As such, they clarify that it is possible to estimate the CMR content and concentration of the syringe as a complete medical product by analysing the product components alone, providing a clear pathway for compliance with the MDR.
At a broader level, the study underlines Stevanato Group’s integrated support for pharma partners. Through its Technology Excellence Centers, the company offers analytical services to secure the generation of objective data to support its customers’ choices. In particular, Stevanato Group’s Chemical Analysis Area is focused on mitigating the risk of chemical interaction with drugs. Thanks to its multi-analytical approach, the company helps to select the proper container closure systems at each stage of the drug development journey to bring medical products to market that meet regulatory demand and satisfy the universal objective of enhancing − and not jeopardising − patient wellbeing.
REFERENCES
-
- “Biomonitoring Summary”. Centers for Disease Control and Prevention, Apr 2017.
- Venkatraman V, “Cobalt-Induced Toxicity and Spasticity Secondary to Hip Arthroplasty: Case Report and Review of the Literature”. Cureus, 2020 Dec, Vol 12(12), Article e12368.
- “IARC monographs evaluate the carcinogenicity of cobalt, antimony compounds, and weapons-grade tungsten alloy”. International Agency for Research on Cancer, accessed Dec 2023.
- “Document 32017R0745. Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC (Text with EEA relevance)”. EUR-Lex, Apr 5, 2017.

