To Issue 138
Citation: “Technology Showcase: Investigation into the Feasibility of a 2 mL Needle-Free Injector”. ONdrugDelivery, Issue 138 (Oct 2022), pp 58–59.
The current paradigm of injectable drug delivery has stagnated since the early 2000s. Despite recent innovations, such as the European launch of the first electromechanical reusable and connected autoinjector (UCB’s CIMZIA (certolizumab pegol) ava Connect® in early 2021), almost all new biologic drugs are launched with specific and undifferentiated self-injection options – a spring-based mechanical autoinjector or a prefilled syringe (PFS), often with attached safety device. This now-common set of options only serves two classes of patients well.
The autoinjector appeals to patients who wish to avoid seeing a needle and can patiently wait for an injection to complete – albeit with sometimes ambiguous cues. The PFS works well for patients who are not needle-phobic and prefer the control over the injection speed. Outside these two classes of patient, each device technology represents a compromise: patients may have to accept a medication with reluctance; need someone else to administer it for them; or need to seek out non-injectable therapies that may require more frequent dosing, dietary restrictions or a greater risk of serious adverse events (note the recent warnings and use restrictions placed on oral janus kinase inhibitors by the US FDA).1 The lack of enthusiasm that arises from having only suboptimal device options may increase the risk of poor adherence and poor persistence on therapy.
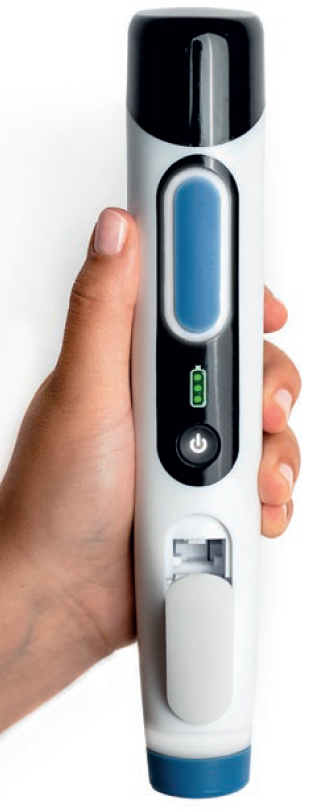
Figure 1: Prime needle-free device.
Additionally, the trend towards larger-volume injections for patients makes these devices even less user-friendly and thus reduces their appeal. This trend is a by product of the need for increased doses for either efficacy reasons or as a way to make dosing less frequent. Typical biologic concentrations are ≤200 mg/mL; as dose needs increase, there is a trade-off between volume and concentration, which leads to either a drug volume or viscosity that results in drug-delivery solutions that either take more time or force to inject. With the trend towards larger-volume injections for patients – 2 mL autoinjectors with similar use characteristics have recently been approved, and there are further studies clinically investigating 2 mL devices – it seems the burden on patients will not lighten anytime soon.
Makers of oral medications in certain classes seem aware of the opportunity created by a suboptimal patient experience and are working to highlight it in their marketing. Pfizer’s branding of Xeljanz® (tofacitinib) as “Unjection™” is the most visible example of this.
Given this context, there is a significant opportunity to transform the patient experience with a reusable and connected needle-free injector. Having already demonstrated in Portal Instruments’ PRECISE II saline self-injection study that the company’s Prime needle-free drug delivery platform (Figure 1) is preferred over a PFS by more than 8:1 (78% versus 9%) amongst healthy volunteers and is reported to inject with lower levels of self-reported pain, the company sought to further explore the platform’s potential with a 2 mL injection. In this study, PIONEER, Portal Instruments explored the feasibility of a 2 mL needle-free injection, the first using a handheld needle-free injection device. The PIONEER study demonstrated similarity in patient-reported outcomes, including perceived pain and user preference, between two 1 mL needle-free injections and a single 2 mL needle-free injection. This demonstrated feasibility of 2 mL needle-free injections and its comparability with two 1 mL doses is exciting for the possibilities it brings to patients.2,3
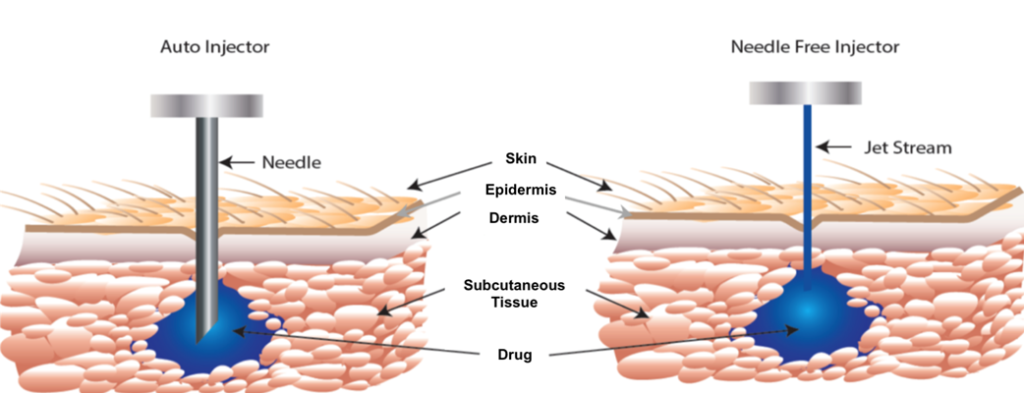
Figure 2: A high-pressure, narrow jet pierces through the epidermis, delivering drug into the subcutaneous space.
First, Portal Instruments’ technology allows for very rapid injection with a decrease in pain perception (Figure 2). A 2 mL injection of a biologic drug may require the patient to press an autoinjector, such as the AJOVY® device (Otsuka Pharmaceutical, Tokyo, Japan), against their skin for 30 seconds. However, Portal Instruments’ platform can inject the same volume in 600 ms, making for a 50-times faster injection. This leads to potential advantages in user preference and reduced risk of user error (e.g. premature lifting of the device during long autoinjector delivery times, resulting in medication loss), with such a significantly faster injection time (Figure 3).
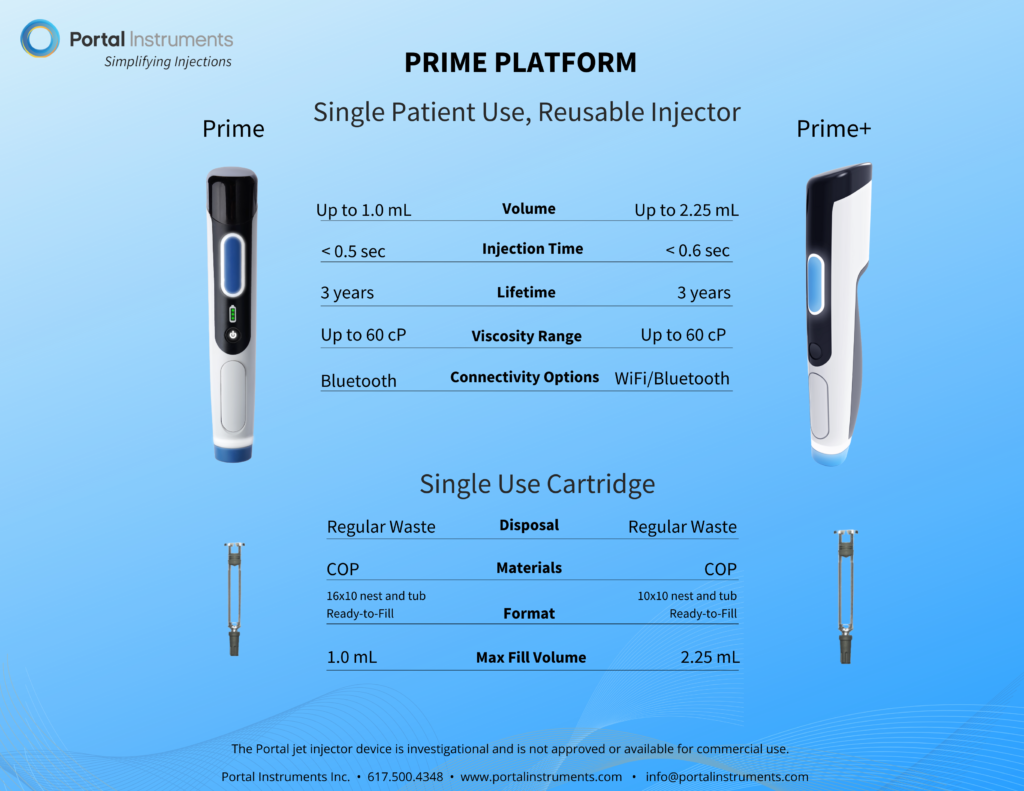
Figure 3: Portal is part of a solution designed to offer next-generation, connected needle-free drug delivery.
Next, the needle-free nature of Portal Instruments’ device may remove a significant barrier to acceptance and adoption of injectable therapies for users who are afraid of needles, which is reportedly 25% of the population.4 Many users, even if they lack needle anxiety, may be reluctant to bring an injectable medication into their home, fearing accidental use by or injury to children or others with whom they reside.
Lastly, the environmental impact of needle-based injections is significant and largely unquantified. A reusable system, such as Portal Instruments’ needle-free injector, which produces less waste in total and eliminates biohazardous waste, would inherently be a better choice for pharmaceutical companies looking to decrease their environmental impact. Altogether, removing disposable needle based devices, which are classified as biohazardous waste, from the world will result in both direct monetary and other societal benefits.
As a fortunate secondary effect, the electromechanical nature of Portal Instruments’ system includes integrated connected health and digital capabilities, which could be used to drive patient adherence and persistence with therapy. Medication non-adherence is a significant drag on the positive and potentially life-changing benefits of all therapies, injectables included.
Note: All trademarks and company names are the property of their respective owners.
REFERENCES
- “FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions.” Drug Safety Communication, US FDA, September 1, 2021.
- Kelley L et al, “Advances in subcutaneous injections: PRECISE II: a study of safety and subject preference for an innovative needle-free injection system”. Drug Deliv, 2021, Vol 28(1), pp 1915–1922.
- Kelley L et al, “Advances in Large Volume Subcutaneous Injections: A Pilot Tolerability Study of an Innovative Needle-Free Injection Platform”. PDA J Pharm Sci Technol, 2022, Epub ahead of print.
- Wolicki J, Miller E, “Vaccine Administration”. US Centers for Disease Control and Prevention, Aug 8, 2021.

