To Issue 138
Citation: Jost R, “The New YpsoMate™ 5.5 – Taking Handheld Self-Injection Beyond Volumes of 2 mL”. ONdrugDelivery, Issue 138 (Oct 2022), pp 8–11.
Reto Jost introduces YpsoMate 5.5, the staked-needle-syringe-based, two-step autoinjector for injection volumes in the 2.0–5.5 mL range, discusses the drivers of the demand for high-volume, high-rate subcutaneous drug delivery using handheld autoinjectors and explains the design rationale of this new injection device.
“Pushing the limits of today’s injection devices has the potential to provide significant benefits to patients, providers, payers and pharmaceutical companies.”
AUTOINJECTORS FOR THERAPEUTIC PROTEINS
The success story of autoinjectors for the subcutaneous administration of therapeutic proteins began in 2006 with the introduction of devices for Amgen’s Enbrel (etanercept) and AbbVie’s Humira (adalimumab).1 With the increasing number of biologic and biosimilar drug approvals, the number of marketed autoinjectors has grown continuously over the last few years. Today, approximately 300 million prefilled autoinjector devices are sold annually, and this number is increasing. Today’s prefilled autoinjectors are typically platform products, such as the YpsoMate device family – which is characterised by simple push-on-skin needle insertion and automatic initiation of the injection process.
Until 2020, all marketed autoinjectors had a maximum injection volume of 1 mL. Over the last five to seven years, much of the demand for new device innovations for subcutaneously delivered drugs has been dominated by the need to inject larger volume injections. This has spawned demand for larger volume handheld autoinjectors, as well as a new device class – patch injectors. With regard to this demand, Ypsomed has increased its YpsoMate autoinjector family with the addition of two new variants, YpsoMate 2.25 and YpsoMate 2.25 Pro, for standard and more viscous drugs respectively. Two examples of recently approved drugs delivered in YpsoMate 2.25 mL autoinjectors are Teva’s migraine product, Ajovy (fremanezumab), which was approved in 2020, and Novartis’ Cosentyx (secukinumab), which was approved in 2021 for psoriasis. Moreover, Ypsomed is industrialising its YpsoDose patch injector for its first clinical trials, acknowledging the growing interest in larger-volume wearable injectors.
“YpsoMate 5.5 represents the newest member of the YpsoMate autoinjector family, extending the design space of today’s handheld
autoinjectors and enabling the administration of injection volumes in the 2.0–5.5 mL range.”
LARGE-VOLUME, HIGH-RATE HANDHELD INJECTIONS
Pushing the limits of today’s injection devices has the potential to provide significant benefits to patients, providers, payers and pharmaceutical companies. Larger dosing volumes allow larger payloads and less frequent injections, reducing the therapy-burden for patients and caregivers. They offer new options in formulation development and, therefore, foster the transition from intravenous to subcutaneous delivery, enabling a shift from hospital to home administration.
Today’s autoinjectors are capable of injecting volumes of up to approximately 2 mL in around 10 seconds, which are medium-volume, high-rate injections. Recent work on increasing the volumes of autoinjector-based injections has been published in preclinical and clinical studies that explore the new field of large-volume, high-rate injections.2–6 The results of these studies indicate that high-rate injections of volumes beyond 2 mL are feasible, paving the way for the development of autoinjectors for volumes above 2 mL.
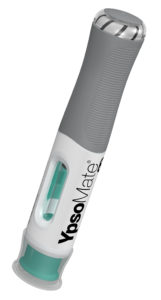
Figure 1: The new YpsoMate 5.5 large-volume autoinjector.
YPSOMATE 5.5 – THE NEW AUTOINJECTOR PLATFORM FOR VOLUMES ABOVE 2 ML
YpsoMate 5.5 represents the newest member of the YpsoMate autoinjector family, extending the design space of today’s handheld autoinjectors and enabling the administration of injection volumes in the 2.0–5.5 mL range (Figure 1). YpsoMate 5.5 is based on the proven YpsoMate 2.25 Pro technology and leverages a similar type of constant force drive mechanism. This ensures that large drug volumes are injected reproducibly, even with higher-viscosity formulations, which would not be possible using a conventional compression spring. Different spring configurations with different force profiles allow the system to be adapted to accommodate a broad range of drug viscosities, up to 30–50 cP. Furthermore, it allows the adjustment of the flow rate to the desired target value. Configurable injection times are in the range of approximately 10–60 s for an injection volume of 5 mL.
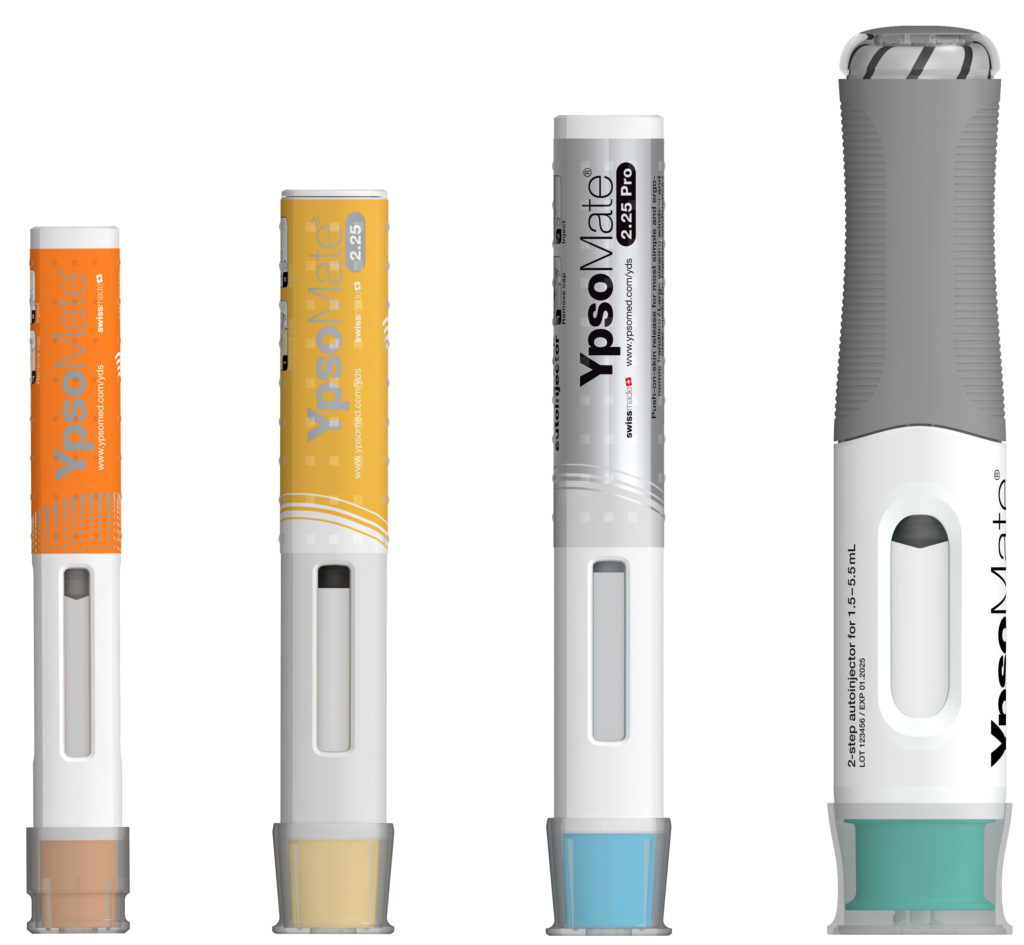
Figure 2: The YpsoMate autoinjector family.
The YpsoMate 5.5 autoinjector features the market-proven and broadly accepted two-step handling principle: the user removes the cap and injects the drug by pushing the device against the skin. As with the other YpsoMate family members (Figure 2), the design is suitable for fully automated manufacturing. YpsoMate 5.5 can be leveraged for a broad range of applications, and offers quick time-to-market and attractive pricing models. The platform design space of the YpsoMate 5.5 is described in Table 1.
| Attribute | Specification |
| Primary container | Staked-needle prefilled syringe |
| Fill volume | 1.5–5.5 mL |
| Viscosity | 1–30 cP, with 27G STW 12.7 mm needle, up to 50 c with larger bore needles |
| Injection time | 10–60 s |
| Needle insertion depth | 5–8 mm |
Table 1: The platform design space of YpsoMate 5.5.
“One of the main goals of the development was to leverage existing standards and components where possible, with the intent to minimise time-to-market, reduce development risks, ensure suitability for sensitive drug products and facilitate compatibility with existing filling lines.”
NEED FOR DEDICATED DESIGN AND USER INTERFACE
Larger-volume injections raise questions about the user’s physical and cognitive capability to hold the device in place during longer injections. Initial evidence for 2 mL autoinjectors confirmed that injection times of up to approximately 30 seconds are feasible in terms of patient’s physical capabilities.7 However, the question remained whether or not long injection times have an impact on the user requirements and whether injection times beyond 30 seconds are feasible.
Therefore, Ypsomed’s development team conducted a series of explorative user studies to assess user capabilities and needs in conjunction with handheld injections of more than 2 mL.
The findings of this research confirmed that even users with medium-to-severe hand impairments were capable of holding an autoinjector in place for injections of up to 70 seconds. Additionally, they indicated that a well-designed grip is essential for convenient handling and a stable hand position during the entire injection. Continuous visual and audible user feedback was preferred over the current “start and end click” feedback for short injections, as well as having plunger-travel visible in the drug window. These findings are designed into YpsoMate 5.5 – the waisted and textured gipping area, along with the contour, allows for a broad variety of grip styles and enables stable handling during the injection. The rotating dial and continuous clicking communicate to the user very clearly the start and the end of the injection, and that the injection is progressing as intended. The design of YpsoMate 5.5 takes into account the particular user needs associated with large-volume handheld injections and supports the user in successfully completing the injection steps, providing the patient with confidence during drug administration (Figure 3).

Figure 3: Enhanced grip design and rotating visual injection indicator.
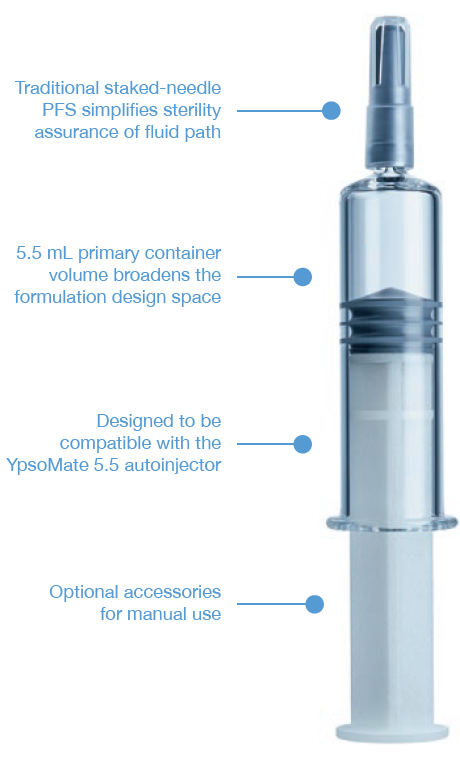
Figure 4: SCHOTT Pharma’s syriQ BioPure® 5.5 mL.
THE STAKED-NEEDLE READY-TO-USE SYRINGE
The staked-needle ready-to-use (RTU) syringe has become the primary container format of choice for use in autoinjectors. The integrated needle with rigid needle shield has a proven track record with respect to container closure integrity and sterility. The pre-sterilised format, packaged in a standard nest-and-tub, is well established and has been successfully industrialised on a wide array of filling lines. Different product presentations are possible based on the same syringe, be it as a stand-alone prefilled syringe (PFS), a safety syringe or an integrated syringe in an autoinjector. Ypsomed has adopted the same prefillable syringe format for YpsoMate 5.5 and, therefore, expanded its existing collaboration with SCHOTT Pharma to develop a large-volume syringe. One of the main goals of the development was to leverage existing standards and components where possible, with the intent to minimise time-to-market, reduce development risks, ensure suitability for sensitive drug products and facilitate compatibility with existing filling lines. The result of this joint development is syriQ BioPure® 5.5 mL, the large-volume prefillable syringe with staked needle for sensitive drugs (Figure 4).
CONCLUSION
With YpsoMate 5.5, Ypsomed extends the limits of current handheld autoinjectors and opens up the new segment of large-volume, high-rate injections in combination with SCHOTT Pharma’s syriQ BioPure® 5.5 mL RTU PFS. As such, YpsoMate 5.5 offers new administration options for biologics in therapy areas such as autoimmune diseases, rare diseases and immuno-oncology.
After successful completion of extensive concept and human factors testing, Ypsomed has initiated a development and industrialisation programme, together with its lead customers, to bring YpsoMate 5.5 into clinical studies. Non-GMP devices and syringes are available for feasibility testing with further drug candidates.
REFERENCES
- Simpson I, “The New Emerging Needs Driving Autoinjector Development”. ONdrugDelivery, Issue 113 (Oct 2020), pp 20–24.
- Shi GH et al, “Subcutaneous Injection Performance in Yucatan Miniature Pigs with and without Human Hyaluronidase and Auto-injector Tolerability in Humans”. AAPS PharmSciTech, 2021, Vol 22(1), p 39.
- Zijlstra E et al, “Impact of Injection Speed, Volume, and Site on Pain Sensation”. J Diabetes Sci Technol, 2018, Vol 12(1), pp 163–168.
- Dias C et al, “Tolerability of High-Volume Subcutaneous Injections of a Viscous Placebo Buffer: A Randomized, Crossover Study in Healthy Subjects”. AAPS PharmSciTech, 2015, Vol 16(5), pp 1101–1107.
- Berteau C et al, “Evaluation of the Impact of Viscosity, Injection Volume, and Injection Flow Rate on Subcutaneous Injection Tolerance”. Med Devices (Auckl), 2015, Vol 8, pp 473–484.
- Tangen LF et al, “The Influence of Injection Speed on Pain During Injection of Local Anaesthetic”. J Plast Surg Hand Surg, 2016, Vol 50(1), pp 7–9.
- Schneider A et al, “Hold the Device Against the Skin: The Impact of Injection Duration on User’s Force for Handheld Autoinjectors”. Expert Opin Drug Deliv, 2020, Vol 17(2), pp 225–236.
Previous article
INDUSTRIALISATION OF CUSTOMISABLE PRODUCT PLATFORMS IN LIFE SCIENCESNext article
SUSTAINABLE DESIGN FOR MEDICAL DEVICES
