To Issue 171
Citation: Eaton J, “The PFAS Problem: Improving Sustainability with Nonwoven Innovation”. ONdrugDelivery, Issue 171 (Apr/May 2025), pp 44–47.
Jack Eaton considers the current landscape for drug delivery and packaging, and how developments and research in nonwoven and fibrous materials can help businesses address the problem of per- and polyfluoroalkyl substances.
Per- and polyfluoroalkyl substances (PFAS) are widely used for their water-, oil- and stain-repellent properties. As one of the strongest single chemical bonds, the carbon-fluorine bond has nearly double the bond strength of a carbon-carbon bond. This means that these bonds resist natural, biological, chemical and microbial degradation. For chemicals containing multiple carbon-fluorine bonds, even if one bond is broken, others may persist – hence the term “forever chemicals” to describe PFAS.
Unintended leakage has led to long-term environmental accumulation, contaminating soil, ground and surface water; disrupting ecosystems; and impacting the food chain (Figure 1). PFAS are pervasive across the human population, thought to be in the blood of nearly every person worldwide.2 The propensity of PFAS to bioaccumulate in the body has already been linked to numerous health issues and chronic diseases, including kidney, liver, bowel and thyroid diseases and cancers; reduced fertility; acute health conditions, such as high cholesterol, that increase the risk of heart attack or stroke; testicular cancer; pregnancy-induced hypertension; pre-eclampsia and small decreases in birth weight; and lower antibody response to some vaccines – which is particularly troubling with a global pandemic still in recent memory.
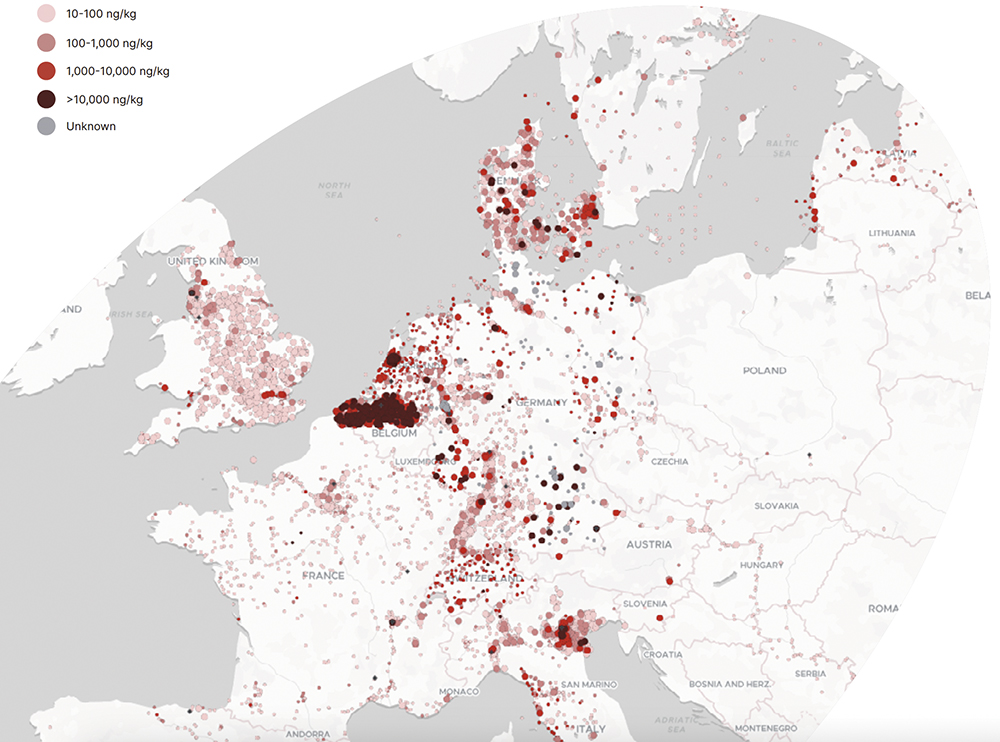
Figure 1: Distribution of PFAS contamination in Europe (adapted with permission from Le Monde and the Forever Pollution Project).1
“AS PUBLIC AWARENESS AND CONCERN GROWS, LEGISLATORS ARE BEGINNING TO TAKE ACTION AGAINST A SITUATION THAT IS NEITHER SUSTAINABLE NOR CONSCIONABLE – THE CLOCK IS TICKING ON PFAS REPLACEMENT.”
As public awareness and concern grows, legislators are beginning to take action against a situation that is neither sustainable nor conscionable – the clock is ticking on PFAS replacement. Within the EU, the European Chemical Agency’s recommendation on the restriction of PFAS will become part of REACH regulations, meaning a total ban on usage of many PFAS above a specified threshold quantity after the transition period. In the US, at the federal level, the Environmental Protection Agency (EPA) has proposed a nationwide ban on the manufacturing and import of certain PFAS. This includes the prevention of manufacture, processing and use of at least three hundred dormant PFAS chemicals that have not been made or used for many years without complete review and EPA risk assessment.
PFAS IN DRUG DELIVERY AND PACKAGING
A recent report from the management consultancy EPPA (Brussels, Belgium) brings the issues facing upstream suppliers into stark relief.3 Some examples of the challenges faced by the drug delivery industry in tackling PFAS are included here.
Blister Packaging
To prevent medication degradation, many solid oral drug packaging solutions – specifically blister packaging – contain PFAS that provide the necessary moisture barrier, and are often transparent to make it easy for patients to identify the medication contained therein. The EPPA report states that “the efficacy and preservation of moisture sensitive drug API is inextricably linked to the barrier performance of immediate drug packaging.”
Drug Delivery Devices
Considering just a small fraction of drug delivery devices – prefilled and reusable pen injectors – PFAS are often key to their component parts. Both types of pen injector incorporate PFAS-containing thermoplastic resins and use PFAS coatings as a dry lubricant. For accurate dosage, particularly to facilitate self-administration, prefilled pens incorporate fluoropolymers to reduce friction and prevent stickiness. Additionally, fluoropolymers are currently crucial to prefilled syringes and devices where a barrier is required between the medicine and the container walls. As the EPPA report notes, “… PFAS are widely used in drug delivery devices ensuring quality and accuracy in the delivered dosing, as well as increasing the lifetime in multi-use devices.”
“FINDING A DIRECT REPLACEMENT FOR PFAS THROUGH ALTERNATIVE CHEMISTRIES PRESENTS A RANGE OF CHALLENGES, BOTH TECHNICAL AND IN RELATION TO THE TIMESCALES OF UPCOMING PFAS LEGISLATION.”
Challenges and Industry Perspectives
Finding a direct replacement for PFAS through alternative chemistries presents a range of challenges, both technical and in relation to the timescales of upcoming PFAS legislation. There is a general consensus that the alternatives that are currently available do not meet all the requirements for safety and performance, and that there are environmental consequences of substitution (both known and not yet known) when investigating like-for-like alternative chemistries.
ADVANCED MATERIAL INNOVATION FOR SUSTAINABLE PFAS ALTERNATIVES
The nonwovens and textile industry is at the forefront of sustainable product development, with applications across a multitude of sectors. These are helping the medical, hygiene and pharmaceutical industries to: navigate sustainability regulations and strategies concerned with single-use plastics and circularity; upgrade product designs; increase recycled, natural and bio-based polymer content; and accelerate the implementation of new recycling technologies. Furthermore, much of this sustainable development is concerned with composite structures with various layers of textile components, coatings and surface functionalisation.
Derogations for PFAS usage in the medical sector are both limited and temporary. Therefore, given the challenges already noted, there is an urgent requirement for an innovative and commercially realistic approach. This is a space where materials innovation can play a role in R&D and product development, working towards PFAS-free alternatives.
Overperformance
Interrogating specifications may provide a solution for some current products where PFAS are routinely used, as some products may be over-specified when considering real-world usage. This is particularly pertinent to new healthcare products, whereby a circular economy approach can be incorporated into the design stage. Decoupling combined requirements for a product, at the earliest stage, may help to ensure that new developments are compatible with circular economy approaches and generate alternative solutions to PFAS. This approach was recommended by MedTech Europe in their recent PFAS position paper.4
“3D THINKING IS AN APPROACH THAT IS PARTICULARLY APPROPRIATE FOR THE DRUG DELIVERY SECTOR, WHERE PFAS ARE PREVALENT IN SUCH A LARGE VOLUME OF COMPONENTS
AND DEVICES.”
3D Thinking
Changing from 2D to 3D thinking means considering the entire product system rather than an individual component or material replacement. 3D thinking is an approach that is particularly appropriate for the drug delivery sector, where PFAS are prevalent in such a large volume of components and devices. Taking a holistic, 3D approach enables different layers and materials to be combined to achieve superior performance without the reliance on PFAS.
As an example, investigating both a woven and a nonwoven PFAS-coated fabric with an oil penetration test, the oil droplet contact angle was seen to be>90 degrees, demonstrating hydrophobicity and avoiding any oil penetration. Focusing on the woven fabric, but with the PFAS coating removed, the oil droplet contact angle reduced from 118 degrees to 58 and then 33 degrees after five seconds. The oil penetrated the fabric sample and had poor oil repellence.
To investigate the potential for PFAS-alternative coatings and laminated structures, two different strategies were evaluated: introducing a PFAS-alternative coating and laminating the woven fabric to a nonwoven. In both of these scenarios, the oil contact angle was improved but neither option was oleophobic, and the oil started to penetrate the fabrics after five seconds. In isolation, then, these strategies did not provide an oil-repellence performance anywhere near to that of the PFAS-coated woven fabric.

Figure 2: By combining strategies and approaches, it is possible to create PFAS-free alternative materials with comparable performance.
However, when the two strategies were combined, evaluating two different types of PFAS-alternative coatings, in both cases the oil droplet contact angle increased and one sample became oleophobic. While the oil eventually penetrated both samples, it became clear that combining variables or strategies can enhance performance. Through additional iterative development and tailoring of the PFAS-alternative coating application parameters and the structural properties of the nonwoven, it was proven possible to develop a PFAS-free composite with comparable performance to the original PFAS-coated woven fabric (Figure 2).
In the space where like-for-like alternative chemistries are not proving suitable for the removal of PFAS from drug delivery devices and pharmaceutical packaging, a holistic, 3D approach can impact the entire product system, rather than just rethinking individual components.
COLLABORATION AND MEETING REGULATORY DEMANDS
The timescales for development of new PFAS-free drug delivery devices and packaging are generally significantly longer than the likely derogation periods. The EPPA report estimates that for solid dose blisters that contain a PFAS layer – assuming that a safe and appropriate alternative can be found – the timeframe is potentially between six and twelve years. The report highlights the logistical issues, where packaging-related testing capacities may well be overwhelmed, given the sheer volume of products that are impacted.
Furthermore, in-house pharmaceutical industry R&D resources are already stretched, particularly in the current volatile economic climate. As MedTech Europe’s 2023 PFAS position paper notes, “Human resources that would otherwise be dedicated to treating new disease states, seeking solutions for new patient populations and solving unmet clinical needs would likely be displaced to research in PFAS-free alternatives for existing products.”
“GIVEN THE VOLUME OF DRUG DELIVERY DEVICE AND PACKAGING PRODUCTS AND COMPONENTS INCORPORATING PFAS, COLLABORATION WITH EXTERNAL INNOVATION SPECIALISTS CAN BE COMMERCIALLY AND ENVIRONMENTALLY VALUABLE, DERISKING IN-HOUSE R&D AND SPEEDING UP NEW PRODUCT DEVELOPMENT PROCESSES.”

Figure 3: Partnering with a materials expert can provide access to the facilities and expertise required to prototype, test and refine PFAS-free materials.
Given the volume of drug delivery device and packaging products and components incorporating PFAS, collaboration with external innovation specialists can be commercially and environmentally valuable, de risking in-house R&D and speeding up new product development processes. To address the question of resource limitation – including the potential for bottlenecks – recourse to external experts can overcome these barriers to the development of PFAS-alternative materials. Where materials innovation can contribute to the removal of PFAS from drug delivery products and packaging, access to prototyping facilities and the capacity to test and refine product developments on-site can narrow the gap between concept and prototype, accelerate R&D, reduce commercial risk and help maintain downstream competitiveness (Figure 3).
CONCLUDING THOUGHTS
The issue of PFAS-free drug delivery devices and packaging is a current and pressing issue, regardless of whatever form of derogations and exceptions from upcoming legislation can be negotiated. However these play out, the clock is ticking on PFAS removal, and short- and long-term strategies are vital. Most companies want, and need, to address public concerns and demonstrate their commitment to environment and social governance strategies. Stringent regulations and lengthy lead-times for approvals of potential alternatives to PFAS create a great deal of uncertainty across the sector.
Whatever regulatory frameworks dictate, through 2025 and the coming years, PFAS are an issue that must be addressed globally. There are clear sector calls for derogation and exceptions, but it cannot be guaranteed that these will include a significant proportion of drug delivery devices or pharmaceutical packaging. To find alternative solutions, across this complex supply chain, collaborating with an external partner is a compelling proposition, helping to reduce commercial risk, speed up time to market with novel solutions and to achieve the ultimate – and necessary – goal of PFAS-free medication packaging and drug delivery devices.
REFERENCES
- Dagorn et al, “‘Forever pollution’: Explore the map of Europe’s PFAS contamination”. Le Monde, Feb 2023.
- Salvidge R, Hosea L, “UK failing to match EU in fight against ‘forever chemicals’, say scientists”. The Guardian, Jan 2025.
- “SOCIO-ECONOMIC ANALYSIS Of the potential restriction of the per- and polyfluoroalkyl substances (PFAS) used in the production, packaging and delivery of human medicinal products”. EPPA, Aug 2023.
- “MedTech Europe Position on the Proposal for A REACH Universal PFAS Restriction”. MedTech Europe, Sep 2023.

