To Issue 144
Citation: van Oorschot B, Smit M, “Unlocking the Potential of Microneedle Technology”. ONdrugDelivery, Issue 144 (Mar/Apr 2023), pp 12–15.
Bart van Oorschot and Maarten Smit discuss the exciting potential of microneedle-based delivery systems to transform the drug delivery industry, the challenges faced by this technology and how the use of an applicator may be the way to overcome them.
“VIPS ranked microneedle technology as the highest priority among drug delivery technologies, considering them to be truly ‘transformational’ innovations that have the potential to overcome many of the barriers to immunisation identified by low- and middle income countries.”
THE POTENTIAL OF MICRONEEDLES
Microneedle technology has the potential to revolutionise drug delivery, diagnostics, cosmetic procedures and a whole range of other applications. Microneedles are tiny needles, mounted on a plate and usually measuring less than a millimetre in length, which can be used to penetrate the skin or other biological membranes. Unlike traditional hypodermic needles, which can cause pain and discomfort, microneedles are designed to be painless and non-invasive and have the potential to significantly improve patient compliance.
Furthermore, microneedles are so small they cannot cause much in the way of damage or injury. This will result in fewer needlestick injuries and fewer infections. They also make it possible for patients to self-administer their therapy, which can have a transformative impact on the costs involved with the infrastructure needed for in-clinic administration by a healthcare professional.
Another benefit of using microneedles is the possibility to eliminate the need for cold chain storage and increase the shelf life of medical products. With most vaccines, for example, the need to keep them refrigerated is a serious strain on the cost and speed of distribution. The importance of this factor was painfully demonstrated during the covid-19 pandemic, in which the costs for distribution and infrastructure were incredibly high, not to mention the impact felt by countries that lack adequate medical infrastructure for large-scale vaccination due to the high costs involved.
The potential of microneedle technology has not gone unnoticed. Recently, some of the largest non-governmental organisations – the WHO, Bill & Melinda Gates Foundation, Gavi, PATH and UNICEF – have joined forces to develop a single integrated framework to prioritise vaccine product innovations and to drive innovations forward called the Vaccine Innovation Prioritisation Strategy (VIPS).1 VIPS ranked microneedle technology as the highest priority among drug delivery technologies, considering them to be truly “transformational” innovations that have the potential to overcome many of the barriers to immunisation identified by low- and middle-income countries.
“The variation in penetration efficiency for manual application varied from about 30%–80% whereas, when the subject used an applicator, the penetration efficiency was about 100% practically every time.”
THE CHALLENGE OF CONSISTENCY
Before microneedles can be used for medical purposes, however, there are a couple of challenges that must be met, one of which is the certification of a complete drug delivery product. It is important to use the final drug delivery method in clinical trials early on. The final efficacy of the medication will be of the utmost importance in these trials, and a key factor that might influence the efficacy is the proper insertion of the microneedles into the skin. If the microneedles are not inserted correctly, it may result in the drug not being delivered to the intended target, leading to reduced efficacy, or even failure, of the treatment. The importance of consistent and effective application was underlined by the rejection of the clinical trial results of Qtrypta (zolmitriptan), a migraine medicine developed by Zosano Pharma (CA, US),2 which ultimately led to the company declaring bankruptcy.3 Qtrypta was rejected because the API exposure levels were too inconsistent.
Additionally, the small and non-invasive nature of microneedle technology also provides a serious challenge to consistent delivery. This is because, in the form that microneedles are usually produced, they are not very easy to use, let alone suitable to be used by anyone.
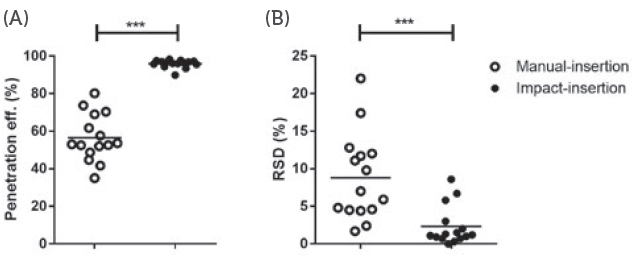
Figure 1: The difference between impact insertion using an applicator and manual insertion.
HOW CAN MICRONEEDLE PENETRATION BE MADE MORE CONSISTENT?
Research conducted by the University of Leiden (Netherlands) demonstrated the difference in consistency of microneedle application when performed with and without an applicator.4 As can be seen in Figure 1, the variation in penetration efficiency for manual application varied from about 30– 80% whereas, when the subject used an applicator, the penetration efficiency was almost 100% every time. This demonstrates that providing patients with an applicator can be a very effective way to improve the consistency and effectiveness of microneedle-based drug delivery. By using an applicator, several variables can be controlled, which normally would reside in the hands of the user.
Probably the most important factor to control is the velocity of the impact when the microneedles hit the skin or the force with which the microneedles are pressed into the skin, depending on which of the two primary methods of insertion is being used. Impact application is more effective but requires a more elaborate applicator, which makes it more suitable for a reusable applicator (Figure 2).

Figure 2: The uPATCH™ Plus, a patented reusable applicator that enables any user to reload the device with a new microneedle patch and consistently and effectively apply it to their skin.

Figure 3: The uPATCH™ One, a patented single-use applicator that combines all functionality in one single shape, minimising material use and environmental impact while still retaining consistency and effectiveness of microneedle insertion.
On the other hand, when sufficient pressing force can be delivered to make sure that the microneedles penetrate consistently and effectively, a single-use applicator can be used (Figure 3).
The researcher Mara Leone investigated how the impact velocity or pressing force with which the microneedles are inserted directly relates to the efficiency of the penetration.5 She inserted six different models of microneedles with varying velocities and pressing forces. Figure 4 shows that these different models have different ideal insertion parameters. By determining the parameters that create the most optimal penetrationefficiency for a specific model of microneedles, the applicator can be optimised accordingly, ensuring the ideal impact or force with as little strain on the user as possible.
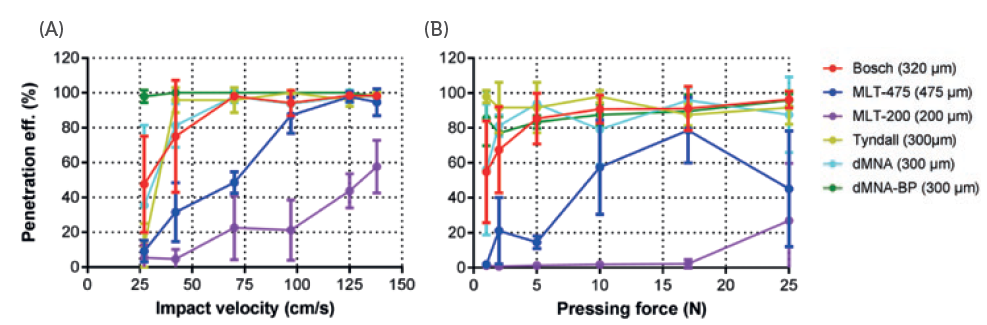
Figure 4: The difference between penetration efficiency with various impact or pressing forces for different microneedles.
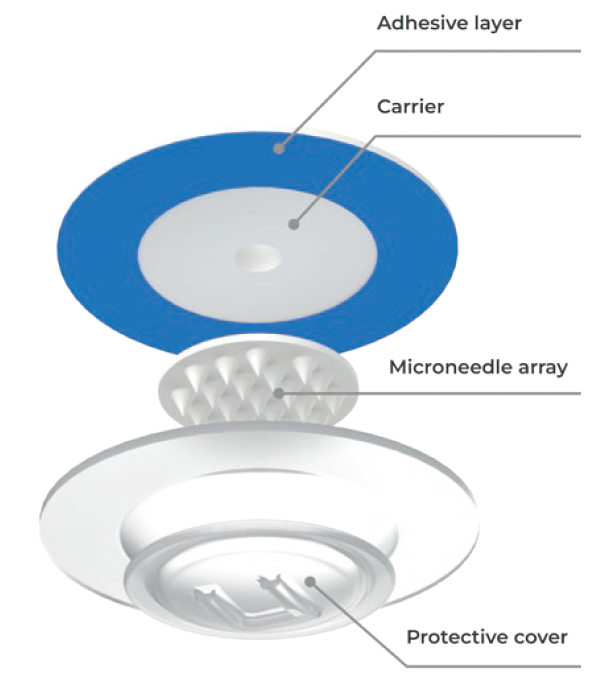
Figure 5: The uPATCH™ microneedle patch consists of a carrier, cover and adhesive layer. This patented patch makes it possible to apply a microneedle array without ever touching it. The cover also protects the microneedles prior to application. The dimensions and simple design of the patch allow practically any type and size of microneedle technology to be accommodated on it.
Optimising the parameters for microneedle insertion is a critical step in minimising the impact on the user’s skin and avoiding undesirable outcomes, such as redness, inflammation or bruising. Not only is this important for enhancing the user’s experience, but it is also crucial for ensuring the success of clinical trials. For instance, in the clinical trial conducted by Zosano Pharma, more than 95% of participants experienced adverse effects at the application site.6 By identifying and using the most optimal parameters for microneedle insertion, the user experience can be enhanced and the efficacy of clinical trial products improved.
As the saying goes, “Variety is the spice of life”, but this variability can be a challenge when it comes to microneedle-based drug delivery. Human beings come in all shapes, sizes and colours, and these differences can greatly affect the efficiency of microneedle penetration. Age, sex, ethnicity and even body fat percentage can all play a role in determining how well microneedles penetrate the skin. However, with the uPATCH microneedle applicator, this variability is taken into account. The device includes legs that gently push on the skin to stretch it out, ensuring consistent penetration efficiency regardless of the individual patient’s physical characteristics. A patch is then applied to keep the skin in position, allowing the microneedles to remain in the skin for as long as necessary (Figure 5). This innovative approach helps to eliminate variability and optimise microneedle-based drug delivery.
CONCLUSION
The development of a reliable and effective microneedle applicator has the ability to unlock the full potential of microneedle technology and revolutionise drug delivery. By eliminating the need for cold chain storage and bringing the point of care directly into the user’s home, this technology can significantly reduce distribution costs and improve access to healthcare. The potential of microneedle technology is vast and, by harnessing its power, the drug delivery industry can create a more equitable and accessible healthcare system for all. With continued research and development, it is possible to look forward to a future where, regardless of location or socioeconomic status, drug delivery is available for every person on Earth.
REFERENCES
- “The Vaccine Innovation Prioritisation Strategy (VIPS)”. Gavi, Jun 2021.
- Kansteiner F, “Zosano presses on after FDA rejects Qtrypta migraine patch”. Fierce Pharma, Oct 2020.
- Taylor NP, “Zosano goes bankrupt after FDA rejects migraine drug delivery patch”. Fierce Pharma, Jun 2022.
- van der Maaden K et al, “Impact-insertion applicator improves reliability of skin penetration by solid microneedle arrays”. AAPS J, 2014, Vol 16(4), pp 881–684.
- Leone M et al, “Universal Applicator for Digitally-Controlled Pressing Force and Impact Velocity Insertion of Microneedles into Skin”. Pharmaceutics, 2018, Vol 10(4), Article 211.
- Nahas SJ et al, “Long term safety, tolerability, and efficacy of intracutaneous zolmitriptan (M207) in the acute treatment of migraine”. J Headache Pain, 2021, Vol 22(1), Article 37.

