Citation: Hasegawa H, Suzuki T, “Update on OXYCAPT™ Multilayer Plastic Vial and Syringe”. ONdrugDelivery, Issue 123 (Aug 2021), pp 29–34.
Hiroki Hasegawa and Tomohiro Suzuki discuss the advantages of OXYCAPT™ multilayer plastic vial and syringe, which combine the benefits of cyclo-olefin polymer with excellent oxygen and UV barrier properties. In particular, the authors focus on recent studies that highlight OXYCAPT’s very low levels of extractables.
“MGC has developed OXYCAPT™ multilayer plastic vial and syringe, which provides the myriad advantages of COP along with high oxygen and UV barrier properties.”
Compared with five years ago, the use of plastic has become much more popular in the pharma industry. As a result, more and more customers have started considering plastic vials or syringes. Although glass used to be considered the best option to protect drugs from oxygen and other negative factors, some critical issues have been pointed out with it, such as delamination, pH shift and breakage. In particular, these problems are especially prevalent with protein-based drugs, such as biologics and gene and cell therapies, that are stored at low or ultra-low temperatures.

Figure 1: The OXYCAPT™ multilayer plastic vial and syringe.
To avoid these problems, cyclo-olefin polymer (COP) vials and syringes are sometimes used for such biologics. COP has some excellent features – including very low levels of extractables, low protein adsorption, high break resistance and excellent pH stability – but it is obvious that its oxygen and ultraviolet (UV) barrier properties are very poor. To overcome the situation, Mitsubishi Gas Chemical (MGC) has developed OXYCAPT™ multilayer plastic vial and syringe, which provides the myriad advantages of COP along with high oxygen and UV barrier properties (Figure 1).
OXYCAPT™ OVERVIEW
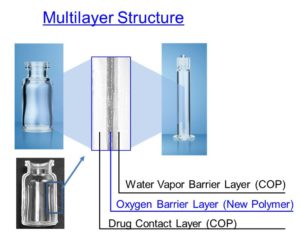
Figure 2: Multilayer structure of OXYCAPT™.
OXYCAPT™ consists of three layers – the inner layer in contact with the drug product and the outer layer are made of COP, while the middle oxygen barrier layer is made of MGC’s novel polyester (Figure 2). Thanks to this multilayer structure, OXYCAPT™ is able to provide the following key benefits:
- Excellent oxygen barrier
- High water vapour barrier
- Excellent UV barrier
- Very low levels of extractables
- High pH stability
- Low protein adsorption and aggregation
- Silicone-oil free barrel
- High transparency
- High break resistance
- Easier disposability
- Light weight.
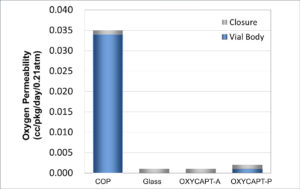
Figure 3: Comparison of the oxygen barrier properties of glass, COP, OXYCAPT-A and OXYCAPT-P.
There are two variants of OXYCAPT™ available – OXYCAPT-A and OXYCAPT-P. OXYCAPT-A has achieved a glass-like oxygen barrier. According to some of MGC’s internal studies, OXYCAPT-A can keep a lower oxygen concentration in its headspace than Type I glass, thanks to its oxygen absorbing function. While OXYCAPT-P lacks an oxygen absorbing function, it provides an excellent oxygen barrier; the oxygen barrier of OXYCAPT-P Vial is about 20 times better than that of a COP monolayer vial (Figure 3).
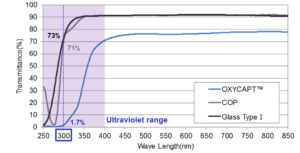
Figure 4: Comparison of the percentage of 300 nm UV light transmitted through Type I glass, COP and OXYCAPT™.
OXYCAPT™ also has excellent UV barrier properties. For example, although about 70% of 300 nm UV light transmits through glass and COP, only 1.7% of 300 nm UV light transmits through OXYCAPT™ (Figure 4). MGC has confirmed that this feature contributes to the stability of biologics.
However, when it comes to acting as a barrier to water vapour, OXYCAPT™ cannot reach the performance of glass. However, its properties in this regard are similar to those of COP, which has seen extensive use in primary containers for injectable drugs and easily meets the water vapour barrier requirements set out by ICH guidelines.
MGC conducted a pair of studies to demonstrate OXYCAPT’s extremely low levels of extractables. The first study was conducted to confirm volatile, semi-volatile and non-volatile impurities from OXYCAPT™. Five solvents – distilled water, 50% ethanol, NaCl, NaOH and H3PO4 – were selected, and impurities were measured by gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-UV spectroscopy-mass spectrometry (LC-UV-MS) after 70 days at 40°C. Compared with the control, no impurities were detected in any of the OXYCAPT™ containers. The second study was conducted to confirm inorganic extractables from OXYCAPT™. The level of extractables demonstrated was similar to that typical of COP, which is well-known as an extremely pure polymer, and lower than that of Type I glass.
OXYCAPT™ vial and syringe are produced by co-injection moulding technology. Although this technology has been used in the production of beverage bottles for many years, MGC is the first company to succeed in applying it for the production of multilayer plastic syringes. MGC has also developed inspection methods for testing the oxygen barrier layer. All of the containers are 100% inspected by state-of-the-art inspection machinery.
“The target therapeutic application of OXYCAPT™ is biologics. As ICH Q5C mentions, oxidation is one of the primary causes of protein instability. As such, the high oxygen and UV barrier properties of OXYCAPT™ contribute directly to the stability of biologics.”
CURRENT PRODUCT OFFERING
MGC can offer bulk vials, ready-to-use (RTU) vials and RTU syringes. Regarding the RTU products, vials and syringes are provided in ISO-standard nest and tub formats. The nest and tub are primarily sterilised using gamma radiation. There are 2, 6, 10 and 20 mL sizes for vials, and 1 mL long and 2.25 mL sizes for syringes (Table 1). MGC is happy to provide samples for initial testing free of charge.
| Type | Volume | ISO | Parts | Option |
| Vial | 2 mL | ISO 8362-1 | Vial | Bulk or RTU |
| 6 mL | ISO 8362-1 | Vial | Bulk or RTU | |
| 10 mL | ISO 8362-1 | Vial | Bulk or RTU | |
| 20 mL | ISO 8362-1 | Vial | Bulk or RTU | |
| Syringe | 1 mL Long | ISO 11040-6 | Barrel, Tip Cap, Stopper, Plunger Rod |
RTU |
| 2.25 mL | ISO 11040-6 | Barrel, Tip Cap, Stopper, Plunger Rod |
RTU |
Table 1: MGC’s OXYCAPT™ product portfolio.
Each polymer meets the requirements set out by United States Pharmacopeia (USP) <661>, USP <87>, USP <88> and all relevant European Pharmacopoeia (EP) regulations. Furthermore, each polymer has been filed in the US FDA’s drug master file (DMF). The vials and syringes also comply with the USP and EP, and have FDA DMF numbers. Regarding the syringes, the products are produced and controlled in accordance with ISO 13485.
“The latest studies have shown an outstanding characteristic of OXYCAPT™ – extremely low levels of extractables.”
The target therapeutic application of OXYCAPT™ is biologics. As ICH Q5C “Stability of Biotechnological/Biological Products” mentions, oxidation is one of the primary causes of protein instability. As such, the high oxygen and UV barrier properties of OXYCAPT™ contribute directly to the stability of biologics. Customers have also recently started evaluating OXYCAPT™ vials for their gene and cell therapies. MGC’s RTU vials and syringes sterilised by gamma radiation are ideal for these protein-based drugs.
In addition, MGC believes that OXYCAPT™ is well suited to adrenaline, as it is well-known to be an oxygen-sensitive drug. Furthermore, as glass syringes suffer more from breakages than plastic ones, they are inherently less suitable for emergency drugs. As such, some suppliers are investigating plastic for the development of new pen injectors for emergency applications.
EXTRACTABLES STUDY FINDINGS
The latest studies have shown an outstanding characteristic of OXYCAPT™ – extremely low levels of extractables. An organic and elemental extractables study, which was performed in collaboration with an outsourcing pharmaceutical company, was conducted using MGC’s container closure system (CCS) comprised of a 1 mL long OXYCAPT-P syringe, custom-ordered PTFE laminated butyl rubber stopper, and a normal butyl rubber tip cap (Figure 5).
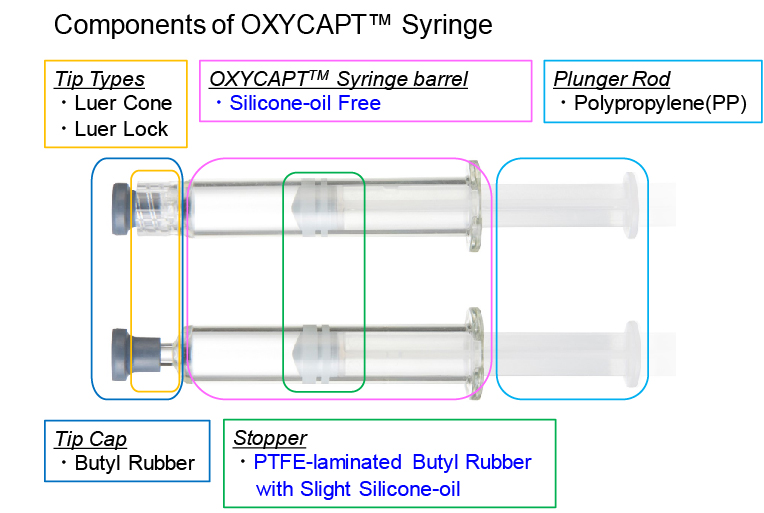
Figure 5: Components of an OXYCAPT™ syringe.
In order to detect and quantify organic analytes, volatile compounds were measured by headspace gas chromatography-mass spectrometry (HS-GC-MS). Moreover, non-polar, volatile and semi-volatile compounds were measured by GC-MS, whereas LC-UV-MS was used to detect compounds with varying polarity and volatility. Furthermore, with the OXYCAPT™ CCS, levels of the elemental impurities specified in ICH Q3D plus tungsten were detected and quantified by inductively coupled plasma mass spectrometry (ICP-MS).
These studies were conducted by a 48-hour incubation at 50°C of the OXYCAPT™ CCS with four different formulations:
- 0.1% aqueous polysorbate 20 (PS-20)
- 0.1 M phosphate buffer at pH 3
- 0.1 M phosphate buffer at pH 10
- 50% ethanol solution.
These extraction solutions cover those outlined in the latest draft of USP <665>. After incubation, a sample work-up for each solvent and analysis method was performed. Then it was confirmed whether there were any organic extractables that exceeded the analytical evaluation threshold (AET) using analytical devices. Each AET of HS-GC-MS, GC-MS and LC-UV-MS was determined by calculation with respect to the safety concern threshold (1.5 μg), filling volume of the syringe, maximum number of syringes administered per day and the concentration factor due to sample work-up. The results of analysis with HS-GC-MS, GC-MS and LC-UV-MS all showed no organic compound exceeded the AET with the OXYCAPT™ CCS (Table 2).
| Analytical method |
Solution | AET (ppm) |
Compounds found above AET |
| HS-GC-MS | 0.1% PS-20 | 0.3 | No compound detected above AET |
| pH 3 buffer | |||
| pH 10 buffer | |||
| GC-MS | 0.1% PS-20 | 1.5 | No compound detected above AET |
| 50% ethanol | |||
| pH 3 buffer | |||
| pH 10 buffer | |||
| 50% ethanol by speed vac | |||
| LC-UV-MS | 0.1% PS-20 | 1.5 | No compound detected above AET |
| 50% ethanol | |||
| pH 3 buffer | |||
| pH 10 buffer | |||
| 50% ethanol by speed vac |
- AET was decided by calculation with reference of butylated hydroxytoluene (BHT) for GC-MS and LC-UV-MS or toluene for HS-GC-MS.
- As to 50% ethanol, the different sample work-up procedures were conducted to target different polarities.
- In case of ‘50% ethanol’ in ‘Solution’, extraction was conducted in sample work-up to target non-polar and volatile compounds.
- In case of ‘50% ethanol by speed vac’ in ‘Solution’, evaporation was conducted in sample work-up to target polar and less volatile compounds.
Table 2: Results of an organic extractables study using the OXYCAPT™ syringe CCS.
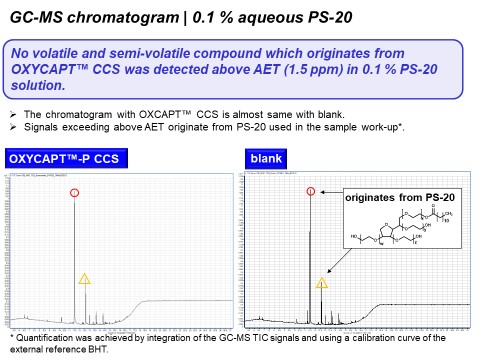
Figure 6: GC-MS chromatogram of OXYCAPT™ CCS and control with 0.1% aqueous PS-20 solution.
Figure 6 shows the GC-MS chromatogram with 0.1% aqueous PS-20. As shown in this graph, some peaks that originate from PS-20 used in the sample work-up exceeded the AET (1.5 ppm) in both the OXYCAPT™ CCS and control. However, there was no peak originating from OXYCAPT™ CCS above the AET. Furthermore, Figures 7 and 8 show the LC-UV and HS-GC-MS chromatograms for 0.1% aqueous PS-20, respectively. As is the case with GC-MS, there were no peaks originating from the OXYCAPT™ CCS detected in either analysis. Moreover, there were no peaks originating from OXYCAPT™ CCS detected with 0.1 M phosphate buffer pH 3, 0.1 M phosphate pH 10 or 50% ethanol either.
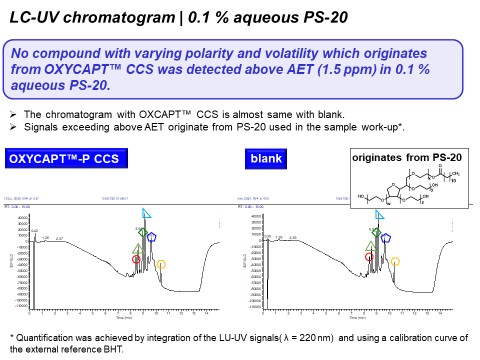
Figure 7: LC-UV chromatogram of OXYCAPT™ CCS and control with 0.1% aqueous PS-20 solution.
In the study with ICP-MS, no elemental impurities exceeded their permitted daily exposure (PDE) as outlined in ICH Q3 (Table 3). Tungsten, which is not listed in ICH Q3D, was not present at a significant level. This result indicates that OXYCAPT™ can contribute to a safety assessment for drugs and the pH stability of drug solution.
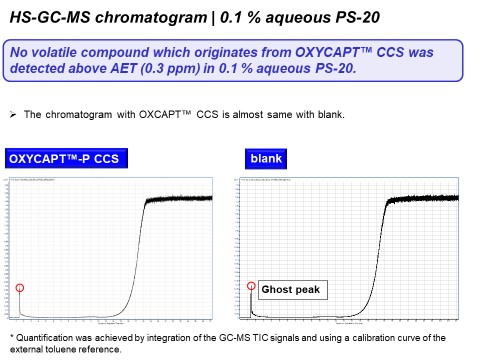
Figure 8: HS-GC-MS chromatogram of OXYCAPT™ CCS and control with 0.1% aqueous PS-20 solution.
| Element | 0.1% PS-20 solution | pH 3 buffer solution | pH 10 buffer solution | PDE (μg) | |||
| Concentration | Amount administered with 5 syringes (μg) |
Concentration | Amount administered with 5 syringes (μg) |
Concentration | Amount administered with 5 syringes (μg) |
||
| Ag | < 0.1 | < 0.5 | < 0.1 | < 0.5 | < 0.1 | < 0.5 | 10 |
| As | < 0.05 | < 0.25 | < 0.05 | < 0.25 | < 0.05 | < 0.25 | 15 |
| Au | < 0.05 | < 0.25 | < 0.05 | < 0.25 | < 0.05 | < 0.25 | 100 |
| Ba | < 0.1 | < 0.5 | 0.1 | 0.5 | 0.1 | 0.5 | 700 |
| Cd | < 0.01 | < 0.05 | < 0.01 | < 0.05 | < 0.01 | < 0.05 | 2 |
| Co | < 0.01 | < 0.05 | < 0.01 | < 0.05 | < 0.01 | < 0.05 | 5 |
| Cr | < 0.05 | < 0.25 | < 0.05 | < 0.25 | < 0.05 | < 0.25 | 1100 |
| Cu | < 0.1 | < 0.5 | < 0.1 | < 0.5 | 0.1 | 0.5 | 300 |
| Hg | < 0.05 | < 0.25 | < 0.05 | < 0.25 | < 0.05 | < 0.25 | 3 |
| Ir | < 0.1 | < 0.5 | < 0.1 | < 0.5 | < 0.1 | < 0.5 | 10 |
| Li | < 0.01 | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 | 250 |
| Mo | < 0.05 | < 0.25 | < 0.05 | < 0.25 | < 0.05 | < 0.25 | 1500 |
| Ni | < 0.05 | < 0.25 | < 0.05 | < 0.25 | < 0.05 | < 0.25 | 20 |
| Os | < 0.1 | < 0.5 | < 0.1 | < 0.5 | < 0.1 | < 0.5 | 10 |
| Pb | < 0.01 | < 0.05 | < 0.01 | < 0.05 | < 0.01 | < 0.05 | 5 |
| Pd | < 0.01 | < 0.05 | < 0.01 | < 0.05 | < 0.01 | < 0.05 | 10 |
| Pt | < 0.05 | < 0.25 | < 0.05 | < 0.25 | < 0.05 | < 0.25 | 10 |
| Rh | < 0.01 | < 0.05 | < 0.01 | < 0.05 | < 0.01 | < 0.05 | 10 |
| Ru | < 0.01 | < 0.05 | < 0.01 | < 0.05 | < 0.01 | < 0.05 | 10 |
| Sb | < 0.01 | < 0.05 | < 0.01 | < 0.05 | < 0.01 | < 0.05 | 90 |
| Se | < 0.5 | < 2.5 | < 0.5 | < 2.5 | < 0.5 | < 2.5 | 80 |
| Sn | < 0.05 | < 0.25 | < 0.05 | < 0.25 | < 0.05 | < 0.25 | 600 |
| Ti | < 0.01 | < 0.05 | < 0.01 | < 0.05 | < 0.01 | < 0.05 | 8 |
| V | < 0.01 | < 0.05 | < 0.01 | < 0.05 | < 0.01 | < 0.05 | 10 |
| W | < 0.1 | < 0.5 | < 0.1 | < 0.5 | < 0.1 | < 0.5 | n.a. |
Table 3: Concentration of each elemental impurity listed in ICH Q3D plus tungsten in a study with ICP-MS.
CONCLUSION
In addition to the existing data, the latest study results showed that OXYCAPT™ CCS has an excellent resistance to aqueous surfactant, acid, alkali and alcohol solutions. As such, OXYCAPT™ CCS, along with its other well-established benefits, can contribute to the safety and efficiency of biopharmaceuticals and gene and cell therapies owing to its extremely low level of organic extractables and elemental impurities.

