To Issue 128
Citation: Müller P, Mächler S, “YpsoMate On – Go for Simply Connected”. ONdrugDelivery, Issue 128 (Dec 2021), pp 34–38.
Philippe Müller and Silas Mächler discuss the benefits of integrated digital therapy management systems and the role of YpsoMate On – the newest addition to Ypsomed’s connected device portfolio, and the world’s first prefilled autoinjector with integrated connectivity that automatically logs injections on the user’s therapy management app.
Medication non-adherence is one of the principal reasons why patients do not achieve expected treatment outcomes. Solving this challenge is a major goal for healthcare organisations. However, studies indicate that 50–60% of chronically treated patients still miss doses, take the wrong dose or discontinue treatment in the first year.1
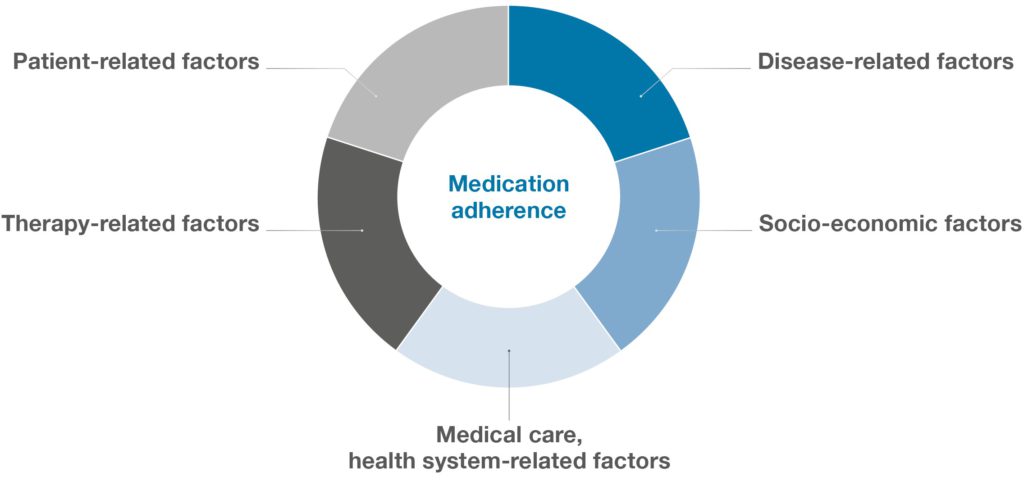
Figure 1: Five dimensions of medication adherence.
Medication non-adherence is the result of a complex interaction of five sets of factors, as illustrated in Figure 1:
- Disease-related factors
- Socio-economic factors
- Health system-related factors
- Therapy-related factors
- Patient-related factors.
“Connected device data can serve as a key input for digital therapy management applications.”
Accordingly, any intervention to improve adherence needs to target the relevant adherence barrier and be tailored to the specific needs of the individual and therapy context.2
Connected injection devices, in combination with digital health interventions, enable new forms of integrated device-and-digital solutions to address the challenge of medication non-adherence and non-persistence, thereby aiming to improve treatment outcomes.
VALUE ADD OF CONNECTED INJECTION DEVICES FOR THERAPY MANAGEMENT

Figure 2: Value of connected devices for therapy management.
The benefits of connected injection devices fall into three categories: advanced user guidance, treatment evaluation and intervention design, as illustrated in Figure 2.
First, connected devices provide additional visible and/or audible feedback directly on the device to guide the user through the injection process. Specific sensors can detect use errors, and smart labelling enables the connected device to check drug expiry date and potential recall. Second, injection data provide important information to healthcare professionals for treatment effectiveness evaluation. Several studies have shown that self-reported adherence data is upward biased as patients overestimate their adherence, leading to inappropriate dose escalation by the healthcare professional.3 Having precise electronic adherence data is key to personalising the treatment schedule and dosage.4,5
Third, injection data can be used for intervention design, such as self-monitoring, prompts and rewards to reinforce adherent behaviour. Interestingly, studies indicate that just capturing and displaying adherence data for self monitoring has a positive effect on medication adherence, for example in growth hormone treatment.6 Further, feeding injection data into digital therapeutics solutions allows for timely and targeted interventions.
These just-in-time adaptive interventions aim to provide the right type and amount of support, at the right time, by adapting to an individual’s changing internal and contextual state.7 The contextual state, for example, includes the detection of use errors by the connected device that then triggers a push notification adapted to the type of use error and the specific user profile. Another example is providing positive feedback or a reward directly after a successful injection. Accordingly, ongoing data collection is required to decide whether the individual requires support, what type of support and whether there is potential to change behaviour.7
As illustrated in Figure 3, connected injection devices may play a key role for just-in-time adaptive interventions as they report an individual’s state in terms of therapy adherence and correct use of the device, and serve as an interface to directly communicate with the user via visual or audible feedback. As such, connected device data can serve as a key input for digital therapy management applications. In addition, the injection data points allow for an evidence-based evaluation of intervention effectiveness in terms of improved medication adherence and persistence.
Looking ahead, new connected device technologies are emerging that focus even more on user-centric design to ensure they are easy and intuitive to use. To achieve this aim, Ypsomed has been working on the development of YpsoMate On, the first prefilled connected autoinjector.
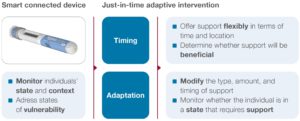
Figure 3: Leveraging injection data for just-in-time adaptive interventions.
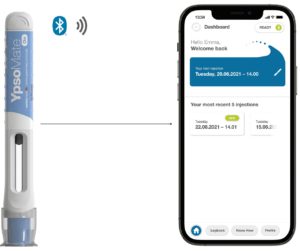
Figure 4: YpsoMate On – the prefilled connected autoinjector.
YPSOMATE ON – GO FOR SIMPLY CONNECTED
“YpsoMate On is the world’s first prefilled autoinjector with integrated connectivity that automatically logs injections on the user’s therapy management app.”
YpsoMate On is the newest addition to Ypsomed’s connected device portfolio. It is the world’s first prefilled autoinjector with integrated connectivity that automatically logs injections on the user’s therapy management app (Figure 4). This new autoinjector adds connectivity and retains the market-proven two-step handling of the YpsoMate platform. As such, YpsoMate On provides connectivity without compromising ease of use. The key features of YpsoMate On are described below in more detail.
Automated Data Capture and Injection Guidance
YpsoMate On consists of a sensor and electronic module that is built into the top end of the device. The sensor captures the start and end of injection and transfers this data via Bluetooth to the user’s smartphone. Furthermore, the LED-based visual feedback allows the user to track the injection progress, including the holding time.
Innovative Device-to-Smartphone Connection Without Pairing
The device-to-smartphone connection is automatically established by Bluetooth proximity measurement, similar to the working principle of covid-19 contact tracing apps. This form of Bluetooth protocol enables secure data transfer without active pairing of the injection device and the smartphone. The device transmits an encrypted report, and the system decides on the validity of the report in the background. If the report is valid, the user receives a new entry in the injection logbook with information about drug product and dosage, as well as time and date of the injection. The injection logbook can then be shared with the healthcare professional and/or caregivers for therapy management and treatment evaluation.
Sustainable Design
YpsoMate On is designed with a focus on separation of the electronics and battery for re-use or recycling. The electronics and battery can be easily detached from the autoinjector, as they are compactly integrated into the top end of the device and do not directly interfere with the inner autoinjector mechanics. The separation process may be performed directly by the user or by a dedicated recycler. Furthermore, YpsoMate On uses sustainable materials and optimisations along the supply chain to minimise the environmental footprint of the device, for example, by using chemically identical bio-based plastics for the majority of the plastic device parts.8
Leverage the YpsoMate Platform
YpsoMate On builds on the market-proven YpsoMate platform. It includes the same spring-driven drug delivery mechanism for reliable and effortless injection. The newly added connectivity module includes a sensor to capture the start and end of the injection without requiring any modification to the core drug delivery mechanics. YpsoMate On is therefore compatible with existing YpsoMate manufacturing and end-assembly lines. This allows for rapid scale-up and short time to market. This also allows existing YpsoMate customers to move to a connected autoinjector without significant new investments in final assembly and packaging infrastructure.
YPSOMED’S CONNECTED DEVICE PORTFOLIO TO SERVE DIFFERENT USE CASES
To serve different market needs and use cases, Ypsomed has developed a portfolio of connected injection devices. The portfolio can be divided into two broad device categories:
- Connected add-ons, such as SmartPilot for YpsoMate
- Injectors with integrated connectivity, such as YpsoDose and YpsoMate On.
Comparing SmartPilot for YpsoMate with YpsoMate On, there are similarities as well as key differences between the two device categories (Figure 5). SmartPilot is the re-usable connected add-on for YpsoMate. SmartPilot captures injection events, detects use errors and provides comprehensive real-time injection support, including drug authentication at point of use and step-by-step guidance. YpsoMate On provides a narrower feature set, retains the proven two-step device handling and enables automated data capture. Moreover, the device includes LED-based visual feedback signalling ongoing injection and completion of the injection, including the holding time.
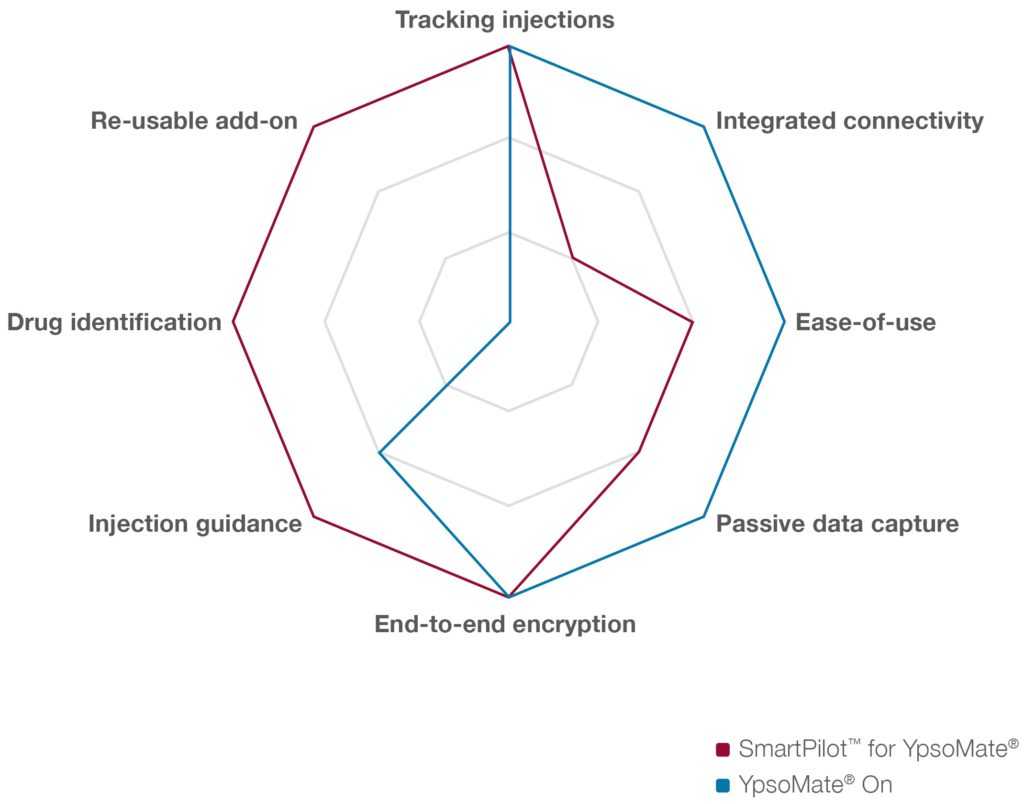
Figure 5: Complementary feature set to serve different patient needs and use cases.
SmartPilot and YpsoMate On represent two different device categories and feature sets that target different user needs.Ultimately, the device choice depends on the intended use of the overall system as well as on the specific user population, injection frequency and other indication specific factors.
CONCLUSIONS
Integrated digital therapy management systems that consist of connected injection devices, a digital therapeutics app and biomarkers offer great potential to improve medication adherence and, ultimately, therapy outcomes. As such, close collaboration between device manufacturers and digital therapy management providers is needed to design effective just-in-time interventions adapted to the specific needs of the individual.
With YpsoMate On, Ypsomed extends its connected device portfolio with a prefilled autoinjector with integrated connectivity. The key differentiating factor is ease of use as YpsoMate On maintains the two-step handling process. Ypsomed offers a growing portfolio of connected device technologies, including SmartPilot for YpsoMate and the YpsoDose connected patch injector. The optimal device choice depends on the drug product, the intended use and the specific user population. Looking ahead, Ypsomed continues to focus strongly on the user-centric development of next-generation drug delivery devices and services to fulfil the needs of evidence-based digital therapy management systems.
REFERENCES
- Hichborn J et al, “Improving patient adherence through data-driven insights”. McKinsey, Dec 14, 2018.
- Sabaté E, “Adherence to long-term therapies: evidence for action”. World Health Organization, 2003.
- Guedes VG et al, “Comparing objective and self‐reported measures of adherence in haemophilia.” Haemophilia, 2019, Vol 25(5), pp 821–830.
- Bittner B et al, (2019). “Connected drug delivery devices to complement drug treatments: potential to facilitate disease management in home setting.” Med Devices, 2019, Vol 12, pp 101–127.
- Vrijens B, “Strategy to Monitor Adherence” in Feldman SR et al, “Treatment Adherence in Dermatology” (Feldman SR et al). Springer International Publishing, 2020, pp 13–19.
- Koledova E, Tornincasa V, van Dommelen P, “Analysis of real-world data on growth hormone therapy adherence using a connected injection device”. BMC Medical Informatics and Decision Making, 2020, Vol 20(1), pp 1–7.
- Nahum-Shani I et al, “Just-in-Time Adaptive Interventions (JITAIs) in Mobile Health: Key Components and Design Principles for Ongoing Health Behavior Support”. Ann Behav Med, 2018, Vol 52(6), pp 446–462.
- Gerner S, Schneider A, “Paving the Way to Zero Carbon Emission Combination Products: Insights From the Ypsomate Zero Case Study”. ONdrugDelivery, Issue 112 (Sep/Oct 2020), pp 56–59.

