Citation: “Interview: Paul Allsop, Miles Kottman, Hannah Priestly and Jon Reed, Consort Medical”. ONdrugDelivery Magazine, Issue 96 (Apr 2019), pp 36-40.
Paul Allsop is a Senior Development Engineer for Consort Medical’s Product Engineering team. He has 22 years’ experience innovating drug delivery devices, for which he has a number of patents to his name. He joined Consort in 1997 and has held roles of increasing responsibility within the R&D function. During his time with Consort Medical he has primarily been involved in the design and development of pMDI valves and actuators, including dose counters.Mr Allsop also designed the Unidose Xtra spring powered nasal device with its unique patented trigger mechanism.
Miles Kottman, Alliance Manager at Consort Medical, has more than 25 years’ experience in the development and industrialisation of medical devices. His career began in in vitro diagnostics. As a programme manager, he led multiple inhalation device programmes from early-stage product development through to high-volume production. Then, as Head of Programme Management at the Bespak division, he was responsible for the programme portfolio and its new-product-introduction process. Now operating in a commercial role, he manages commercial partnerships for existing and new products and opportunities. He is responsible for commercially supporting customers with the Unidose Xtra nasal delivery device.
Hannah Priestley is Graduate Commercial Manager at Consort Medical having joined the company’s Graduate Scheme in September 2018 after graduating from The University of Warwick (UK) with an MSc in Medical Biotechnology and Business Management. Her role involves learning all aspects of the commercial function, including analysis of key market segments including nasal, injectable and respiratory.
Jon Reed, Business Development Director at Consort Medical, has more than 15 years’ experience, in both healthcare and pharma, including managing complex solutions into the pharma industry. Mr Reed has an MBA specialising in business strategy and is responsible for commercially supporting customers with Consort Medical’s Unidose Xtra nasal delivery device.
In this interview with ONdrugDelivery Magazine, this group of colleagues from Consort Medical discuss the company’s integrated formulation and device offering, and focus on its simple, low-cost, single unit-dose, spring powered nasal device, Unidose Xtra.
“Consort’s full value proposition to customers … means that we not only have the device itself, but we can formulate API to go into that device, we can manufacture, assemble and package the device, and supply that to the end-customer, streamlining the supply chain.”
Q Could you give an overview of Consort Medical’s broad offering and business structure, in particular in light of the rebranding last year?
MK Consort Medical is a developer and manufacturer of drugs and premium drug delivery devices. Globally, we employ around 2,000 staff and we’re committed to investing in patient- clinical- and customer-driven innovations to create new treatments. We offer customers a single source for drug and device development, formulation, manufacture, fill and finished form, with two integrated operational divisions – Bespak and Aesica.
We partner with pharma companies with our core business providing innovative, life-improving treatments to patients across the world through two integrated activities. One is the design, development, and manufacturing of high-performance drug delivery devices such as, injectables, nasal, and ocular delivery systems as well as point-of-care diagnostics. The other integrated activity is the development, formulation, and manufacture of active pharmaceutical ingredients (APIs) and finished-dose drugs to the highest quality standards.
In summary, Consort Medical offers a one-stop service to streamline the development of pharmaceuticals, and their route to market.
The Bespak division was founded in 1959 so we have more than 50 years’ experience in drug delivery. We work with customers across a full spectrum from the pharma and biotech industries, that are developing specialist drugs through to branded generics.
Today, we make in excess of half-a-billion devices annually, mainly through our King’s Lynn (Norfolk, UK) site.
We make over two-and-a-half billion moulded components annually, so we specialise in high-volume, high-complexity injection moulding and complex device assembly. We’ve also got capabilities for prototyping, and low-volume to high-volume automated production.
JR Turning to the Aesica division, Aesica is a full-service developer and manufacturer (CDMO) of active pharmaceutical ingredients (APIs) and finished dosage forms. We partner with customers to provide a flexible, efficient and dependable service that leverages our innovative approach and more than 30 years’ experience. We continuously invest in the latest technologies and develop our people to stay at the forefront of the industry. As part of the Consort Medical Group, we work together with our drug delivery device experts to accelerate the route to market of drug-device combinations, through streamlined supply, for our customers at any stage of the development cycle.
We have a number of large pharmaceutical company partners and manufacturing sites across Europe, mainly in Germany, Italy and the UK. Our Queenborough site on the Isle of Sheppey (Kent, UK) is one of our largest.
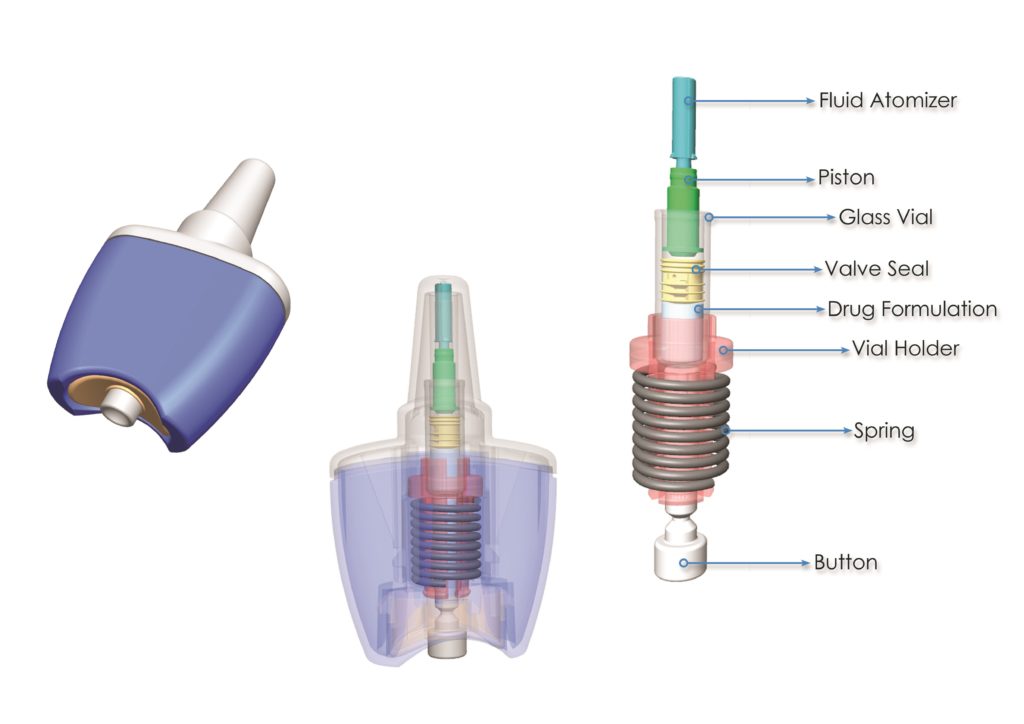
Figure 1: Consort’s single unit-dose, spring powered nasal delivery device, Unidose Xtra.
Aesica’s core competency, combined with that of Bespak, enables Consort to offer a unique end-to-end service to its customers. For example, with the Unidose Xtra, single unit-dose, spring powered nasal delivery device (see Figure 1), this combined offering means that we not only have the device itself, but we can formulate API to go into that device, we can assemble and package the device, and supply that to the end-customer, streamlining the supply chain. The major advantage is that we are able to offer a single-supply-chain solution to customers wanting combination products.
“The nasal route is particularly suitable for delivering drugs that require rapid onset of action.”
Q Let’s focus on nasal delivery now. From the point of view of the demands and requirements of today’s market, what can nasal delivery offer?
MK There is a well-established nasal delivery market across a wide range of therapies. The two types of delivery devices (for aqueous formulations) are multi-dose, pump-like products, and unit dose-type products. The interesting area for us, where we’re observing market growth and recent product approvals, is with unit-dose products. In particular, we’re seeing the repurposing of treatments that have traditionally been administered by injection. Examples of these include therapies for pain management, and emergency lifesaving treatments. New formulations, and reformulations, are emerging and with the inclusion of drug absorption enhancers it is allowing smaller delivered volumes and lower dosages, which is ideal for administration through the nasal cavity. Together with lower cost and simpler devices, these developments can offer many benefits over and above autoinjectors, for pharma companies and the end user.
HP The nasal route is particularly suitable for delivering drugs that require rapid onset of action. It has advantages over both the oral and parenteral routes. Nasal delivery is non-invasive yet fast-acting, reaching the systemic circulation quickly. It avoids first-pass metabolism and there’s potential for direct delivery to the brain. This makes nasal delivery suitable in the treatment of anaphylaxis, pain, migraine and, in particular, opioid overdose. Focusing on opioid overdose, according to the US Centers for Disease Control and Prevention, in the US 47,000 people died in 2017, and currently 130 people a day die, from opioid overdose.
In the treatment of anaphylaxis and opioid overdose, it’s important to remember that it may not actually be the recipient of the drug that’s delivering it. Treatment often requires third-party intervention and the nasal cavity gives good access for quick delivery for a relatively unskilled, untrained individual. So to place a device into somebody’s nostril is relatively simple, especially in an emergency situation.
“We recognise and generate the necessary performance data on our products, which is made available to share with partners for regulatory submissions, significantly reducing their risk burden.”
Q What are the most important patient needs, and how can a nasal delivery system meet these needs?
PA Patients need intuitive, simple devices for any type of application, including nasal. A unit-dose nasal system offers an ideal, simple, one-handed device solution, which is easy to use and small enough to carry around everywhere. This all helps to promote patient compliance. In some cases devices also need to be used by a third-party to administer the drug to a patient without the need for any skill or training.
Users of the device also need flexibility. They don’t want a device where their own interaction interferes with successful delivery. If they have to perform a set of tasks in a very specific way to achieve optimum delivery, that’s not ideal because everybody will do things differently. It is key to have a device that anybody can use, and it doesn’t really matter too much how they use it, they still achieve the same end result, successful drug delivery.
For example, in a standard pump-based nasal device, variability in actuation speed can lead to a difference in spray performance, whereas ideally this should not be influenced by the user. Also, force to actuate is key to achieving optimum delivery. Therefore we have developed a device with a low actuation force and the utilisation of a spring takes care of the required force and velocity element for us. Having a low actuation force makes a device more user-friendly and attractive to a wider variety of users.
In a Laboratory environment where a machine is used to activate these types of devices, variability can be low due to the parameters being used. What we’ve aimed for with our Unidose Xtra device is to provide Laboratory performance in the patients’ hands, so that any variability between users has minimal influence.
That probably can’t be said for some other devices in the marketplace where they require a fixed group of parameters to be used to achieve optimum delivery. Designing this influence of patient variability out of the device’s performance I think is key.
JR Portability is another factor. In anaphylactic shock, for example, some of the key benefits for patients with epinephrine (adrenaline) administered through a nasal spray rather than through a parenteral autoinjector centre around how compact and portable the device is. People who could suffer from anaphylactic shock typically need to carry around an autoinjector wherever they go. Unit dose nasal devices, like the Unidose Xtra, are actually very compact, very portable – they can fit into your pocket.
“Submissions can be
time-consuming, expensive, and risky. Pharma companies are looking for technology that is proven across a number of potential populations, therapies and drugs, with supporting data around robustness and reliability.”
Q What do you see are the pharma industry’s needs when it comes to selecting the best delivery solutions partner? How can a nasal delivery system meet those needs?
JR When developing a product to serve a customer need, there are so many factors that must be considered and I think one of the big advantages that Consort has is that we understand the device as well as actually developing a formulation to go in that device. Pharmaceutical companies are looking for a path to move a project all the way to a finished product, developing the formulation and device performance in unison with technical and regulatory input for their submission.
A key part of our value proposition is that we can undertake the formulation work in our actual devices, or device work in formulations we’ve also developed. This reduces the number of companies involved in bringing a product to market.
Increasingly, pharmaceutical companies are looking to streamline the approach in this way, decreasing time to market.
With its integrated offering, Consort can truly determine the best path forward.
We’re offering both formulation- and device-based solutions and so there’s no vested interest in recommending one or the other. It’s a genuine decision as to what’s truly the best approach to guarantee the best outcomes for the project, based on expertise and capabilities across both device and formulation aspects, and a knowledge of the whole project.
“Beyond designing, developing and supporting regulatory
approval we have a manufacturing platform behind us to
be able to manufacture in line with market demands.”
MK From a partner such as Consort Medical, pharma and biotech companies are looking for the lowest-risk approach for their product and their submission. Device development and drug development submissions can be time-consuming, expensive, and risky. Pharma companies are looking for technology that is proven across a number of potential populations, therapies and drugs, with supporting data around robustness and reliability. We recognise and generate the necessary performance data on our products, which is made available to share with partners for regulatory submissions, significantly reducing their risk burden.
We’ve already mentioned reliability of dosing, closing that gap between what the lab results give you and what actual performance you get, and we’ve talked about a robust, streamlined supply chain solution with fast speed to market.
All of these factors feed into reducing risk.
Our partners can expect a commercially viable product with continuity of supply. Beyond designing, developing and supporting regulatory approval, we have a manufacturing platform behind us to be able to support market demands.
Q How does the Unidose Xtra nasal platform represent an attractive device solution for pharma partners?
PA Unidose Xtra is our single unit-dose, spring powered nasal delivery device. It incorporates patented, needle-free technology.
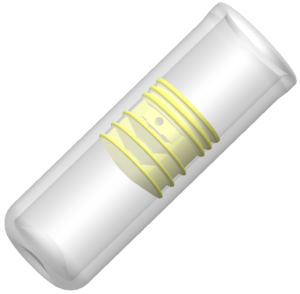
Figure 2: Unidose Xtra’s patented primary pack,
which is made of up the glass vial, the drug, and the seal itself.
We have a patent around the seal technology and how that operates in the primary pack, which is made of up the glass vial, the drug, and the seal itself (Figure 2). As we have a patented springpowered mechanism (Figure 3), Unidose Xtra does not rely on the actuation force or speed being provided by the end user. Being spring-driven like an autoinjector takes away user variability, so Unidose Xtra delivery is completely, 100% controlled by the device itself. The user simply triggers the device by pressing a button and the device does the rest. In addition to simple, low-force, push-button operation, there’s minimal button travel as well. This is all key and means it is suitable for a variety of users. Whether a child is using it or an elderly patient, or even an untrained third party, they’ll all be able to easily trigger the device so that the mechanism can take over and deliver the same dosage at the same rate, irrespective of the user.
Unidose Xtra has an ambidextrous profile. It’s not orientation-dependent, which would be important in an opioidoverdose situation if somebody is horizontal as opposed to somebody standing up or sitting down. So it can be used in any orientation without any impact on performance.
The Unidose Xtra activation button also serves as a use indicator. It moves as you depress it of course, but then as the device triggers and takes over, the button actually moves inside the device, offering a clear indication that it has been used. Therefore having no button visible makes it very obvious to a user that a device has already been used. Obviously, no cleaning or priming is required because it’s single-use.
We have developed a platform device that’s fully adjustable. For example, the spray pattern can be tailored. Pharma companies are looking to differentiate their products, so we can customise the styling, colours, etc. The optimised delivery for the device is set at 100 μL, so it’s targeted around the standard volume that’s typical for these types of nasal devices. Regarding materials selection, we can verify extractables profiles for specific parts and, as with all devices, we use regulatory compliant materials where necessary.
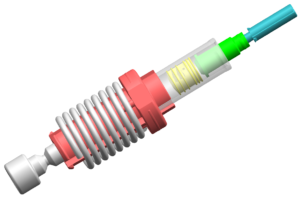
Figure 3: A patented spring-powered mechanism means that Unidose Xtra does not rely on the actuation force or speed provided by the patient or user.
MK With our customer-focused service, we represent a true partner, with the shared objective of delivering the end-solution. From a product development and industrialisation perspective teamed with a robust New
Product Introduction process, we offer an end-to-end service for Unidose Xtra. From formulation development, all the way through to device manufacture, filling, packaging and release.

