Citation: Roested J, “A Patient Centric and Pharma Company Centric Prefilled Wearable Bolus Injector”. ONdrugDelivery Magazine, Issue 97 (May 2019), pp 44-46.
Jesper Roested explains why the pharma/biotech drug development process often means device selection occurs after Phase II despite the advantages of earlier selection being well understood, and how this then precludes the selection of novel primary packaging and delivery systems. He goes on to describe Subcuject’s wearable injector, which meets the requirements of, and does not disrupt, existing pharma development processes and infrastructure.
Over the past few decades, the drug delivery device industry has developed injection solutions to meet the requirements of new biologic treatments coming through pharma pipelines. These devices are patient friendly, with focus on usability, leading to e.g. a number of cost effective and easy-to-use autoinjectors that are prefilled and require no assembly or setting by the patient. Furthermore, disposal of conventional, single-use injection systems presents only a minor challenge as the environmental impact of disposal is minimal.
As the patient experience with an injectable drug is heavily influenced by the delivery device, the advantage of selecting the delivery device for a specific drug early in development is well known in the industry. However, the reality is that the delivery device is often chosen late in the process.
“Devices that are prefilled must be relatively inexpensive – taking up only a few percent of the expected drug sales price in order to fit with the conventional pharma business model – and also allow for
pricing flexibility at a later stage.”
Over the past years, pharma companies have come to focus on biologics as their source of genuine new targets. Whilst the rewards for success are high, there is of course always a high risk with biologics of not meeting ambitious clinical endpoints. Therefore, pharma companies emphasise the advancement of clinical results as early as possible and the choice of the delivery device tends to be pushed until efficacy is indicated in a successful Phase II trial (Figure 1).
As a consequence of this, pharma and biotech companies often conduct their preclinical chemistry, manufacturing and control (CMC) studies using standard materials such as glass and standard rubbers and so when Phase II is successfully completed, and Phase III is about to commence, little time is available to investigate the stability of the drug in other primary packaging materials.
Furthermore, manufacturing must be ramped up during Phase III and primary packaging solutions that do not fit in to existing filling lines would be cumbersome to handle. Therefore, injection devices that are not based on standard materials or which do not fit in to existing filling lines are often ruled out by pharma at the very point in the development process where the decision on delivery system is made.
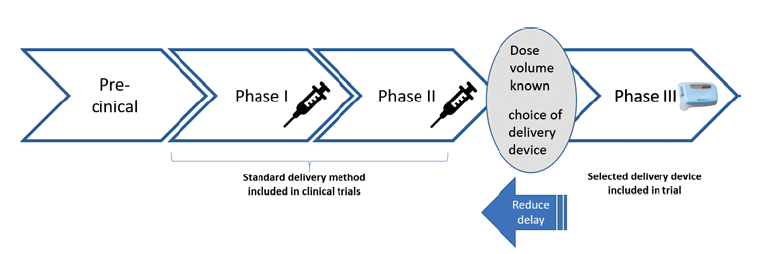
Figure 1: The delivery device is often chosen after Phase II, despite the advantages of earlier selection being well known.
For autoinjectors, device developers have managed to present solutions that incorporate standard primary packaging materials covered by standard CMC packages and that fit in to existing filling lines. Thereby, the hurdle for a pharma company to select such an autoinjector solution prior to Phase III introduces only small additional risk regarding development and manufacturing.
Furthermore, devices that are prefilled must be relatively inexpensive – taking up only a few percent of the expected drug sales price in order to fit with the conventional pharma business model – and also allow for pricing flexibility at a later stage.
Inexpensive manufacturing requires a low number of components, an uncomplicated set-up when it comes to assembly process of the device, and an uncomplicated process at the legal manufacturer (usually the pharma company or filling CMO). Inexpensive manufacturing also requires that sterility barrier solutions do not set requirements for assembly in a sterile environment or require terminal sterilisation.
Eventually, the complete prefilled product must have a shelf life comparable with that of the same drug delivered in a syringe, and the disposal of the single-use device must be acceptable to patients and society in terms of patient/HCP safety and the environment.
“The primary packaging will not pose any drug stability risks due to new materials and its filling may be done on existing filling lines.”
The popularity of wearable larger volume injectors is expected to grow considerably over the coming years and many solutions are in development. The challenge faced by developers of larger volume injectors is to inject the drug slowly and in a controlled manner. Many of the wearable injector systems in development rely on electromechanical solutions and these systems seem to function well in this respect. However, many of the systems require either patient assembly of parts (e.g. due to sterility barriers), or they use primary packaging materials that require new drug stability testing because the materials used were not included in the early drug CMC studies.
Furthermore, the electromechanical and electronic components in such devices result in complex, relatively expensive injection solutions that may be less easily disposed of in an environmentally friendly way after single-use by patients.
Additionally, electromechanical injectors tend to become rather bulky. And finally, they require batteries, which are not the best fit with another requirement of these devices, the ability to remain unaffected by years of cold storage.

Figure 2: Subcuject’s prefilled, single-use, wearable
bolus injector (first version with a 3 mL standard cartridge).
SUBCUJECT’S SOLUTION
Aware that it is not possible or reasonable to ask the entire pharmaceutical industry to transform the way it develops its products.
“Only the needle unit needs to be sterilised and the drug cartridge is filled and sealed in a standard aseptic production environment. The rest of the device does not require sterilisation and no terminal
sterilisation is needed.”
Overall the Subcuject device is smaller than most other wearable bolus injectors, it is fully mechanical and it has a low part count. The only operations to be carried out by the user are to peel off a paper backing, place the device on the body and push a button. Needle insertion and retraction is handled automatically. The device can be adapted to inject even highly viscous drugs at around 1 mL per minute. After use, the device is removed from the body and discarded.Subcuject is developing a prefilled, singleuse, wearable bolus injector for injection volumes below 10 mL (Figure 2), which takes into account all of the requirements outlined above and meets the challenges arising, meaning the pharma industry can select it at any stage of development, without introducing undue additional disruption, cost and risk. The Subcuject wearable is based on an internal osmotic drive unit using a fluid to push the plunger of a standard drug cartridge (Figure 3). It represents a very simple mechanical concept, yet with high drive capacity.
The cartridge used in the device is a standard glass cartridge with a plunger of a standard rubber material and the sealing of the cartridge towards the needle unit consists of the same rubber material as used for the plunger. Thereby, the primary packaging will not pose any drug stability risks due to new materials, and its filling can be done on existing filling lines.
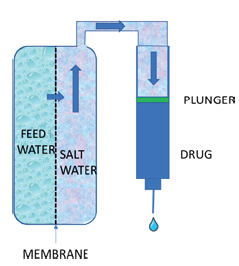
Figure 3: The Subcuject wearable’s
internal osmotic drive unit uses
fluid to push the plunger of a
standard drug cartridge.
The Subcuject device is designed such that the drug in the cartridge and the needle unit both have sterile barriers. Thereby, only the needle unit needs to be sterilised and the drug cartridge is filled and sealed in a standard aseptic production environment.
The rest of the device does not require sterilisation and no terminal sterilisation is needed. The drive fluid does not come in contact with the drug or the patient. The device only consists of relatively few and simple parts and it is designed for automated assembly at a device CMO.
The legal manufacturer (pharma or drug filler) will only need to insert the drug cartridge and click on the housing before release. Thereby, the resulting total cost of goods for the device gets close to the cost of a prefilled autoinjector.
The Subcuject device is designed for not compromising the drug shelf life at cold storage and as the device is fully mechanical, battery discharge is not an issue. Additionally, the absence of electronics means that disposal after single use is no bigger issue than it is for e.g. autoinjectors. Currently the Subcuject device concept for 3 mL drug volume is undergoing performance testing.
CONCLUSION
Subcuject can confidently claim that its wearable bolus injector is just as pharma company centric as it is patient centric. It achieves this by fitting in with the reality of the device selection process of pharma companies, while presenting a very convenient, easy to use wearable bolus injector for patients.

