Citation: Rijcken C, “Company Showcase: Cristal Therapeutics”. ONdrugDelivery Magazine, Issue 97 (May 2019), pp 10-11.
Over the past year, Cristal Therapeutics has transitioned from a research-stage start-up based on a nanoparticle technology platform, to a fully fledged, clinical-stage business with a promising pipeline of proprietary drug candidates. This diverse pipeline together with the proprietary nanoparticle platform, CriPec®, presents a broad range of promising late- and early-stage partnering opportunities for companies active in the oncology space.
The CriPec® platform (Figure 1) forms the backbone for the company’s R&D efforts. CriPec® is built using tailormade, proprietary polymers that Cristal Therapeutics’ expert team of scientists apply to create nanomedicines that transiently entrap a pharmaceutical payload. The resulting CriPec® nanomedicines improve therapeutic performance by shielding the drug from healthy tissues and increasing its exposure to the target tissue. The drug payload is entrapped in the core of the CriPec® nanoparticles using proprietary, covalent linkers that prevent it from escaping the particles and being exposed to healthy cells. The high stability of CriPec® nanomedicines allows them to circulate in the bloodstream for much longer than the native drug molecule can achieve. This long circulation combined with the small size of CriPec® facilitates accumulation in tumour or chronically inflamed tissue via a phenomenon known as the Enhanced Permeation and Retention (EPR) effect. This is the observation that blood vessels in tumours and chronically inflamed tissues are more “leaky” than those in healthy tissue, allowing very small particles like CriPec® to escape through the gaps in the vessel wall and into the tumour interstitial space.
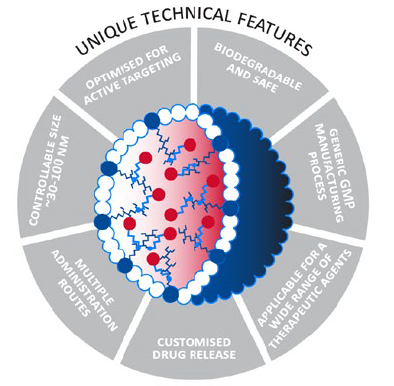
Figure 1: Tuneability of CriPec® platform – CriPec® nanomedicines can be fully tweaked dependent on the indication and the API(s), respectively.
Release of the payload from CriPec® nanomedicines is driven purely by chemical hydrolysis. The rate and site of release can be customised dependent on the needs of the specific payload to further increase tumour targeting and local sustained exposure. This results in a higher ratio of the administered drug reaching its target and in turn a lower ratio being exposed to healthy tissue. This drives an enhanced efficacy and safety profile resulting in a substantially improved therapeutic index.
Cristal Therapeutics’ lead CriPec® nanomedicine programme, CPC634, employs the taxane docetaxel. Whilst docetaxel is an essential standard-of-care treatment across a wide variety of solid tumours, the native drug suffers significantly from various toxicities that limit its use and efficacy in many patients. CriPec® has the unique ability to address these shortcomingswhilst further enhancing the efficacy of docetaxel to provide a vital treatment option for patients, validating the potential of this technology.
Cristal Therapeutics also has several preclinical candidates in its diverse portfolio utilising multiple therapeutic modalities, such as oligonucleotides and peptides, and targeting various tumour types.
CLINICAL EVALUATION
CPC634 was successfully evaluated in a Phase I clinical trial. The data demonstrated that CPC634 is safe and well tolerated at potentially therapeutic doses and has a significantly better pharmacokinetic profile compared with conventional docetaxel. These early-phase results support the basis of tumour targeting via the EPR effect to provide an improved treatment for patients with a variety of solid tumours.
An abstract on this study, “A phase I dose-finding and pharmacokinetics study of CPC634 (nanoparticle entrapped docetaxel) in patients with advanced solid tumours” (poster #3026), will be presented at the American Society of Clinical Oncology (ASCO) Annual Meeting (May 31-June 4, 2019, Chicago, IL, US).
Based on the promising early signs of efficacy in Phase I, CPC634 was advanced to a Phase II trial in October 2018, which is currently ongoing. The trial is evaluating safety, tolerability and efficacy in a well-defined patient population with platinum-resistant ovarian cancer, an indication with no effective therapies currently, and very poor survival rates.
“Several of these additional target indications are
especially prevalent in Asia and the emerging market of China. As a result, the company is proactively seeking partners with proven expertise in successful drug development and commercialisation in Asia.”
Cristal Therapeutics intends to find a partner to expand the CPC634 development programme to further solid tumours including prostate, breast and lung cancers. Several of these additional target indications are especially prevalent in Asia and the emerging market of China. As a result, the company is proactively seeking partners with proven expertise in successful drug development and commercialisation in Asia.
In addition to the ongoing Phase II trial, Cristal Therapeutics is developing a radiolabelled CPC634 to enable non-invasive visualisation of the nanomedicine in patients (Figure 2). This radiolabelled CPC634, known as CPC205, is being tested in a clinical study to demonstrate its tumour accumulation in patients, and provides the basis for the potential future development of a companion diagnostic. An abstract on this study, “First-in-human imaging of nanoparticle entrapped docetaxel (CPC634) in patients with advanced solid tumours using 89Zr-Df-CPC634 positron emission tomography / computed tomography (PET/CT)” (poster #3093), will also be presented at ASCO 2019.
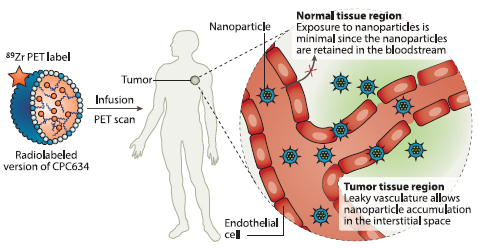
Figure 2: Clinical visualisation of tumour uptake. The radiolabelled CPC634 carries an 89Zr PET label enabling non-invasive imaging by PET/CT scans.
BROAD APPLICABILITY CRIPEC®
Utilising docetaxel enables Cristal Therapeutics to develop its lead candidate in an efficient way with an attractive cost and risk profile that simultaneously demonstrates the benefit of CPC634, and validates the potential of the CriPec® platform as a whole, for other therapeutic payloads. CriPec® is an extremely versatile platform that enables the entrapment of a wide variety of therapeutic modalities including small molecules, peptides and oligonucleotides. Payloads can be entrapped as monotherapies, as is the case for CPC634, or as synergistic combinations to provide further therapeutic benefit.
This broad applicability is driven by Cristal Therapeutics’ ability to customise various aspects of the platform to the specific requirements of particular drugs and diseases. This can be achieved by tailoring the size of the nanoparticles between 30 and 100 nm, tailoring the linker used to entrap the drug, or by using targeting ligands on the surface of the particles to provide even more specific delivery to cancer cells.
PARTNERING WITH CRISTAL THERAPEUTICS
Cristal Therapeutics has several ongoing collaborations with large pharma and biotech companies working on a range of therapeutic modalities and diseases. To capitalise on the full potential of CriPec® the company seeks further partners developing (immuno-)oncology and other drugs that can benefit from CriPec® tumour targeting. Cristal Therapeutics collaborates by first conducting a joint proof-of-concept study to allow partners to test their compounds in combination with CriPec®, followed by a licensing deal on the CriPec® platform and mutual further development.
Partners will benefit from collaborating with an experienced company with proven expertise in the nanomedicine field, a strong intellectual property portfolio, and an established good manufacturing practice (GMP) site that allows straightforward manufacturing at clinical scale. These foundations make Cristal Therapeutics the ideal partner for swift development of tumour targeted nanomedicines.
Through these collaborations together with its own diverse pipeline, Cristal Therapeutics is a dynamic, development-stage enterprise with the expertise to take cancer therapies and other treatments to the next level by allowing drugs to realise their full potential.

