Citation: Tunkel M, Rose C, “User-Centred Device Connectivity”. ONdrugDelivery Magazine, Issue 98 (Jun 2019), pp 54-56.
Mark Tunkel and Carolyn Rose discuss the benefits of taking a user-needs approach, and mapping the patient journey, in a connectivity strategy.
The methods available to pharmaceutical companies and device suppliers to support the patient journey and experience are offering more possibilities than ever before. Traditionally, the focus of the patient experience has been on optimising the “administration task” as outlined in the instructions for use, with an emphasis on the reduction of use error risk. However, as clinical outcomes are increasingly driving payer decision making, developers are expanding their efforts to support the patient journey more broadly. This is being achieved through enhanced methods of training, value-added packaging and longitudinal methods of engagement that span from the onset of a disease state through various life stages.
In addition to these measures, virtually every developer in the branded and generic space is formulating some variation of a digital health strategy that features device connectivity for parenteral and inhalation devices. Initially, these strategies focus on the development of add-ons or accessories that are compatible with a commercially available device and provide users with device performance feedback – like when an autoinjector is ready to inject or when an injection is complete. They can also provide error correction and other means of feedback to help eliminate use errors. The Smart Pilot accessory from Ypsomed is an example of a device providing this level of functionality. These devices are very effective at helping to mitigate potential use error with more mature devices. These devices also feature a mobile application that can collect and curate use information for potential integration into a patient’s health plan.
“There has been convergence within the drug delivery ecosystem, where all aspects of managing a disease state are integrated through connectivity.”
There has also been convergence within the drug delivery ecosystem, where all aspects of managing a disease state are integrated through connectivity. A good example of this is the Bigfoot Biomedical connected diabetes management ecosystem, which is a comprehensive solution that integrates the Abbott Diabetes Freestyle Libre sensor-based glucose management technology into Bigfoot insulin pens and pumps. Abbott has also partnered with Novo Nordisk in a similar arrangement. All these ecosystems are reliant upon sensor technology and mobile applications that act as the primary interface for all the component pieces. Similar to the add-ons scenario, these ecosystems are addressing known workflows and device component constraints.
In the future, it is reasonable to expect that novel and platform device ecosystems will begin to integrate connected technology into the device architecture itself. While there are regulatory issues to address, patient experiences that include connectivity and analytics supported by Amazon Web Services or Google cloud platforms hold immense promise to deliver better outcomes and experiences for patients and health systems.
Achieving success will require the use of a robust digital health strategy. A critical tool in helping developers formulate a user-driven strategy is a thorough mapping of the patient journey – to develop an intimate understanding of foundational user needs so that the entire journey is considered at the earliest stages of development.

Figure 1: Applied ethnography helps developers learn about the context of use and the patient journey in self-administration as the foundation for a connected device strategy.
A comprehensive understanding of the user enables the team to determine how these needs can be most effectively addressed, whether through the device, a software capability or with an interplay between the two augmented by other assets. This approach helps ensure developed technologies are not only meeting actual market needs; they’re doing so in an optimal way – eliminating potential redundancies and conflicts that are inevitable when developing device and software in isolation.
Understanding, characterising and prioritising user needs is best achieved through applied ethnography (Figure 1) which consists of a combination of interviews and observation within the context of use. This method can yield a deep, longitudinal understanding of the patient journey (Figure 2) – from diagnosis to treatment selection, onboarding and ongoing use. With an emphasis on system touchpoints like education/training materials, secondary packaging and device interaction, the patient journey can then be leveraged as a critical early-stage decision-making tool.
Like drug discovery, this type of research should be started as early as possible in the development process – user needs are the cornerstone in defining the path forward. While some believe considerations surrounding the patient and device should be suspended until drug attributes are clearly defined, this approach can lead to decision making on device development or selection that can take a developer too far down a development path to make meaningful changes.
“User needs should be characterised as soon as
there is a known target patient population.”
While usability testing activities are well understood when it comes to device selection and are critical at this stage, discovery research is a fundamentally different type of activity that seeks to define and understand user needs rather than help ensure they are met. User needs should be characterised as soon as there is a known target patient population – to most effectively drive the development of solutions covering the device, connectivity, mobile applications, data analytics and enhanced training that address the whole of the patient journey.
Ultimately, this information can be synthesised into a development road map. This consists of mapping identified needs with corresponding device and supporting element opportunities and capabilities. The road map can then be used to guide early mapping of the ideal envisioned patient and healthcare professional journey, and the process of exploring solutions that best meet the needs at each phase of the journey while enabling the team to consider solutions more holistically.
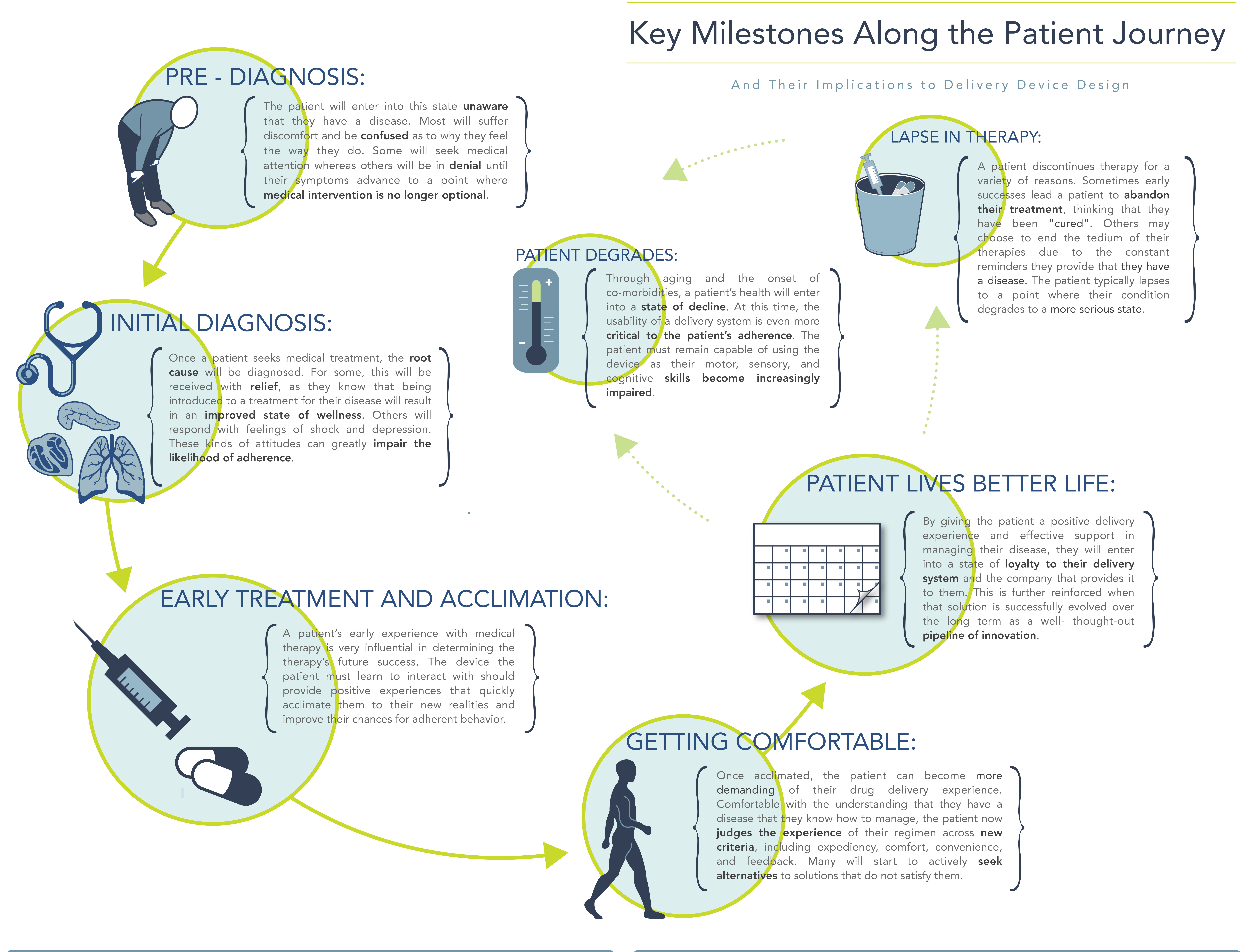
Figure 2: The output from ethnography is a patient journey map that identifies opportunities for improving user experience or mitigating risk, including by using connected solutions.
This is particularly important when determining where the integration of connectivity and data analytics can meaningfully support the experience. The road map also becomes the foundation for later requirements definition for the device, the mobile application and any other clinical interface required, ensuring that requirements stem from user needs, rather than known technical capabilities and limitations.
The benefits of taking a user-needs approach in a connectivity strategy include:
- Helps developers ensure that all elements of a device strategy for a new drug product effectively address user needs optimally and holistically
- Provides early guidance on device selection (for platform devices) or organic device development requirements as well as gaps that need to be addressed by packaging, instructions for use, training and areas where connectivity can be impactful
- Identifies potential areas of user-based risk that may be present in clinical trials and in market so they can be mitigated as early as possible in development
- Works as the basis for developing a data analytics strategy that harnesses the insight into what elements of disease state management are most meaningful and worth of measurement for a particular application.
Ultimately, this approach drives efficiency in development as both commercial and research and development teams can address key portions of the strategy proactively to ensure all meaningful aspects of the patient journey or healthcare provider experience are enabled to support the ideal envisioned care model – and not used as a mitigation to a substandard experience or risk.
This will also benefit either novel or platform devices with connectivity features as a means of commercial differentiation, particularly in biosimilar or generic applications where multiple entrants may be using similar (or identical) devices.

