Citation: Metzmann F, “Developments in Self-Injection Devices for Combination Products”. ONdrugDelivery Magazine, Issue 101 (Oct 2019), pp 92-96.
Fred Metzmann reports on the latest state-of-the-art developments in innovative injection devices for combination products in subcutaneous self-application. He outlines Haselmeier’s product platform strategy for single-use (D-Flex) and re-usable (i-pen²) injection-pen systems, and related services and looks at the company’s connected digital solutions.
“For drugs that require subcutaneous application, self-injection pens offer an ideal opportunity to control the time factor and reduce risk.”
High cost-pressure in the healthcare sector means that to launch a new combination product successfully, a drug must not only be safe and effective, but the combination of drug and device must also guarantee reliable and beneficial therapy and, of course, be economical.
Many biopharma companies are currently looking for solutions that enable rapid drug development, smooth conduct of clinical trials and fast device development for commercialisation of their combination products. For drugs that require subcutaneous application, self-injection pens offer an ideal opportunity to reduce time and risk.
ADVANTAGES OF SELF-INJECTION PENS
Self-injection applications are an effective way to minimise the costs associated with managing and treating a broad spectrum of diseases. This means self-injection pens are increasingly the first choice for new, subcutaneously administered pharmaceutical agents. These biopharmaceutical products are being developed through a combination of industry innovations and new pharma companies.
Self-injection solutions also have the potential to greatly improve the quality of care from the patient’s perspective. Self-administering by the patient – for example, with injection pens – offers several advantages. The patient has more flexibility in terms of place and time of treatment, thereby substantially reducing therapy costs.
Therefore, where it is possible for medication to be administered using an injection pen, the devices should be used as the pharmaceutical form as early as possible in clinical studies.
Generally during the clinical phase, medication is stored in vials and administered to the patient through a disposable syringe. However, vials can be inconvenient to use and harbour safety risks, especially in terms of shelf-life after opening, contamination and injury to staff due to the cannula. An alternative is the prefilled syringe, but this does not permit any dose adjustment and, as such, would mean substantial additional demand for the trial drug. Moreover, common to both vial-and-syringe administration and prefilled syringes is that they are unsuitable for use by non-medical laypeople. Administering a drug once or several times a day would require substantial nursing care potentially including hospital treatment.
“Haselmeier’s new D-Flex product platform strategy offers innovative technical features that enable customers to accelerate product development with a drug agent and delivery pen that are combined during development rather than sequentially, as is traditionally the case.”
DEVELOPMENT OF IMPROVED COMBINATION PRODUCTS
It is important to develop not only pen technology and manufacturing methods, but also focus particularly closely on how to improve the engineering process for new self-injection pens.
Haselmeier is constantly adapting its offerings to meet customers’ needs – in response to the factors that drive their markets and patients. It has always been Haselmeier’s top priority to be a strong, proactive partner, which has been demonstrated with its recently released injection pen system, the D-Flex product platform. Together with a connected digital solution, D-Flex Connect, and a pharma packaging solution, Haselmeier offers a comprehensive service, helping its partners save time and reduce risk in the development and market launch of combination products.
THE D-FLEX PRODUCT PLATFORM STRATEGY
Haselmeier’s new D-Flex product platform strategy offers innovative technical features that enable customers to accelerate product development with a drug agent and delivery pen that are combined during development rather than sequentially, as is traditionally the case.
This makes the D-Flex product platform an ideal, flexible platform for adapting to set doses in accordance with therapy. It has been developed and validated so that only minimal drug- and customer-specific adjustments are necessary (Figure 1). These can be seamlessly integrated into the clinical supply chain process up to serial production following market authorisation.
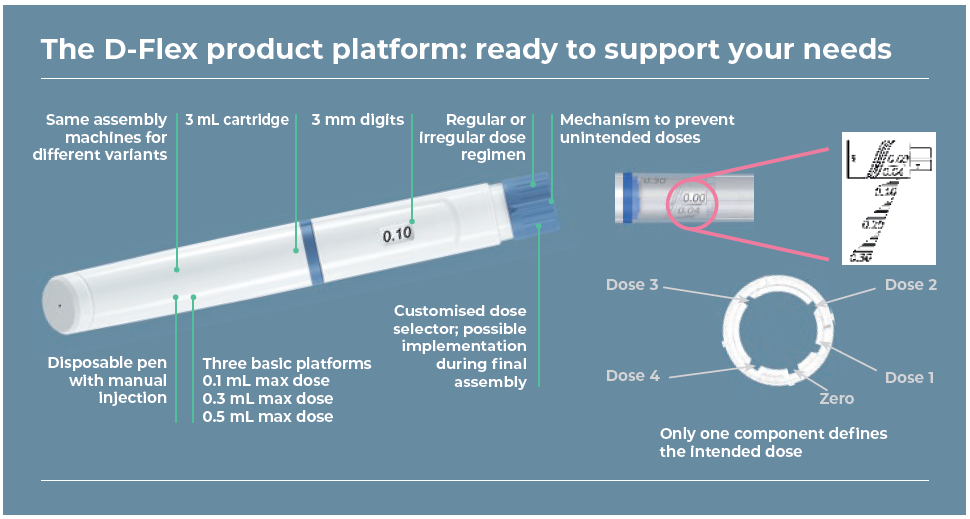
Figure 1: The D-Flex product platform has a range of beneficial features.
Use in Clinical Trials
The requirements of an injection pen for clinical studies are:
- Simple and safe to use, and permits a predefined flexibility in the adjustment of the dose in Phases II and III
- Permits therapy-appropriate labelling, including re-labelling for dose adjustment as well as the option of multilingual labels
- Can be used continuously or with minimum adjustments for all phases of the clinical study up to drug approval
- Can be delivered ready-to-use to trial participants without having to establish in-house manufacturing competence.
The new product platform is therefore highly suitable for clinical trials. The disposable pen for use with 3 mL cartridges can be configured for several fixed doses, bridging the gap between fixed- and variable-dose pens. These dose values can be freely selected when designing the pen. This is especially of interest for dose-escalation studies, for example. The pen system does not allow any intermediate steps between the set doses, significantly reducing the risk of a wrong dose and enhancing patient safety.

Figure 2: The Haselmeier D-Flex pen is designed for use with 3 mL cartridges but can be configured for several fixed doses.
The D-Flex product platform pen can also be quickly and cost-efficiently adjusted to customer needs or their requirements for clinical-trial design, by replacing just one single part of the device. Modifications after Phase II are possible and the drug delivery device can be adjusted to the requirements of other products in the drug development pipeline (Figure 2).
D-Flex can be flexibly configured to suit the desired dose values from the first clinical study to series production, significantly reducing capital expenditure and time-to-market.
THE D-FLEX PRODUCT-SERVICE SYSTEM
The D-Flex Product–Service System (also known as the D-Flex ecosystem) consists of the D-Flex pen with an optional smart cap that enables Bluetooth data collection and transmission as well as data management to an optional platform to develop health management applications. These components are combined with Haselmeier’s engineering services, which support the parallel integration of drug and delivery pen development processes, plus a pharmaceutical packaging service (Figure 3). Haselmeier’s D-Flex ecosystem significantly accelerates timeto- market while simultaneously reducing risks during the platform pens’ initial development phases.
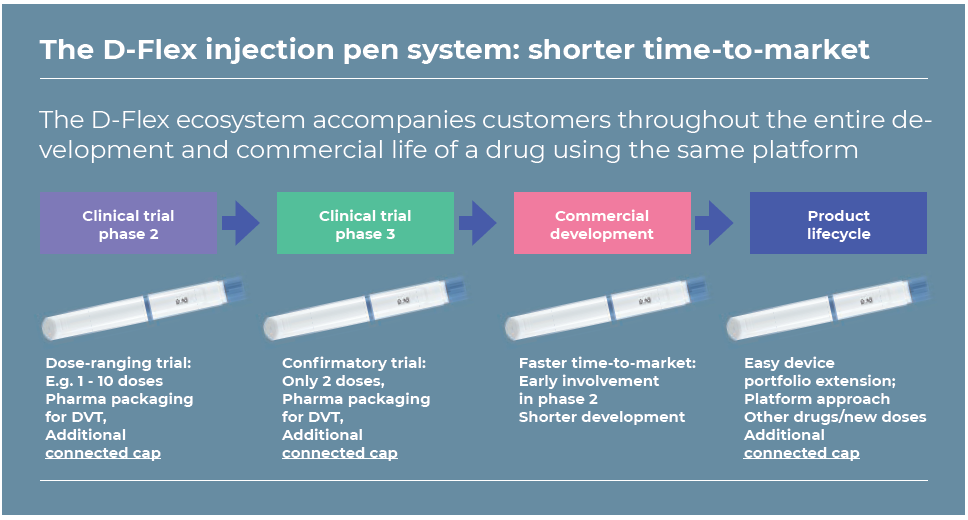
Figure 3: The D-Flex ecosystem comprises the D-Flex pen, an optional smart cap that enables Bluetooth connection (with associated software/apps), and engineering and pharma packaging services.
The parallel development of drug and delivery platform can start as early as Phase II. The disposable pen can be used during trials as it can easily be adapted to different dose requirements and improves the trials’ quality by lowering the risk of manual handling errors and simultaneously reducing the required investment. The pen uses the information and outcomes gathered from the trials to simultaneously adjust and engineer the final delivery pen. This shortens development cycles while minimising the customer’s investment risks.
The D-Flex product platform offers two versions of the final pen solution. Depending on the patient’s needs or the doctor’s prescription, the pen can deliver different doses. The dose can either be continuously adapted, or the correct dose can be selected from a range of presets.

Figure 4: The D-Flex connected digital solution.
DATA MANAGEMENT AND MONITORING IN CLINICAL TRIALS
The option to equip the D-Flex pen with Haselmeier’s smart cap D-Flex Connect enables bio-pharmaceutical partners to collect data at the point of care and improve the quality during clinical trials by monitoring compliance with study design during Phase II and III trials. This makes a sustainable contribution to increasing and demonstrating therapy efficiency. In addition, the smart cap lets you help patients to manage their personal health by combining pen delivery with appropriate user-friendly apps (Figure 4).
The flexibility of the D-Flex injection system together with the pharmaceutical packaging, the possibility to produce small quantities and the D-Flex Connected digital solution make the system a very promising solution for cost saving and time reduction in drug development and improvement of therapy efficiency.
In short, the D-Flex is ready for our pharma partners to apply to their specific application needs – quickly and easily.
THE i-PEN2 PRODUCT PLATFORM
Haselmeier’s i-pen2 product platform solution can help pharmaceutical companies to accelerate time-to-market for their injection pens if this needs to be balanced against cost pressures. This reusable variable-dose injection device is designed for use with standard 3 mL cartridges – and so combines the Haselmeier platform concept with the benefits of an established infrastructure for production and certification processes (Figure 5).
The Haselmeier i-pen² solution was specifically created to provide a high-quality pen at a low economic cost and offers an affordable alternative to fully customised pens. Although it is a platform solution, the i-pen² offers high flexibility and variability in design and appearance. Haselmeier operates a production facility for the i-pen² in India to meet the needs of self-injection patients across the world and applies certified German manufacturing and quality standards.

Figure 5: The Haselmeier i-pen2 is a reusable variable-dose injection device.
ENSURING QUALITY DRUG EVELOPMENT PROCESSES
Quality of care is defined by two key aspects: the latest quality and safety requirements for healthcare products, and patient-centric approaches that address the convenience of products, safe usage and treatment compliance. This is a challenge for market participants throughout the entire product lifecycle. At Haselmeier, customers are supported with quality assurance processes by working closely together with them at every stage – from initial design specifications to user feedback integration, production, product delivery and continuous quality control measures. This carefully co-ordinated approach helps ensure the best possible outcome for the quality of care that patients receive.

