Citation: Suzuki T, “Latest Update on OXYCAPT™ Multilayer Plastic Vial and Syringe”. ONdrugDelivery Magazine, Issue 101 (Oct 2019), pp 102-106.
Tomohiro Suzuki gives an update on the development of the OXYCAPT™ multilayer plastic vial and syringe – highlighting the results of recent tests comparing it with existing glass syringes.
OXYCAPT™ is a multilayer plastic vial and syringe. It consists of three layers (Figure 1): the drug contact layer and outer layer, both made from cyclo-olefin polymer (COP); and the oxygen barrier layer, which is made from a proprietary, novel polyester.
“We have recently decided to invest in a facility for the staked needle syringe. The necessary equipment will be installed during 2020.”
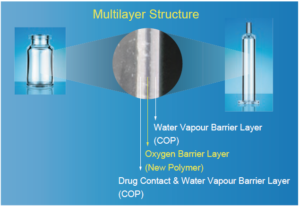
Figure 1: Multilayer structure of OXYCAPT vial and syringe.
Thanks to its state-of-the-art multilayer technology, OXYCAPT™ has excellent oxygen barrier and high water vapour barrier properties, very low extractables, low protein adsorption, excellent ultraviolet (UV) barrier properties, high break resistance, high pH stability and a silicone-oil free barrel.
Although COP is the most promising candidate when it comes to replacing glass with plastic, the oxygen barrier is insufficient for oxygen-sensitive drugs. According to one of our experiments, the oxygen barrier of COP is more than 100 times worse than the existing glass-syringe system. We therefore started developing multilayer plastic vials and syringes about seven years ago to ameliorate the disadvantages of a COP vial and syringe.
To begin with, as a manufacturer of special polymers, we developed a new polymer that has excellent oxygen barrier properties and is suitable for multilayer injection moulding. The new polymer for the middle layer is a kind of polyester filed in US Drug Master Files and compliant with US and European pharmacopoeias.
In parallel with the new polymer, we developed innovative multilayer injection moulding techniques. Such injection moulding technology has been applied to beverage bottles for many years – it contributes to preventing oxidation and carbon-dioxide evaporation of drinks. Our idea was to transfer the technology to the vial and syringe in the pharma industry.
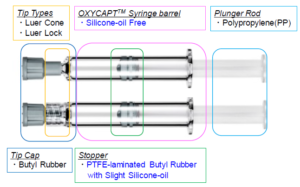
Figure 2: Components of OXYCAPT syringe.
The OXYCAPT syringe consists of several components (Figure 2). There are two types of tips, and the stopper is made of polytetrafluoroethylene (PTFE)-laminated butyl rubber with a small amount of silicone oil. To minimise the protein-aggregation problem (Figure 3) caused by silicone oil, no silicone oil is baked on the inside of the barrel.
We tried to confirm the influence of minimised silicone oil on biologics, and conducted protein aggregation studies. The commercially available antibody was filled into OXYCAPT and Type 1 glass syringes, and the syringes were shaken at 500 rpm for one week at room temperature. A week later, tiny sub-visible particles were measured by resonant mass measurement (RMM), and large sub-visible particles and visible particles were measured by dynamic image analysis(DIA). We found the minimised silicone oil significantly contributes to preventing protein aggregation of the antibody.
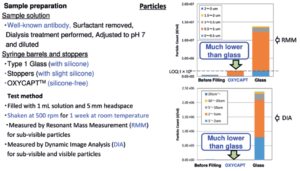
Figure 3: Protein aggregation studies.
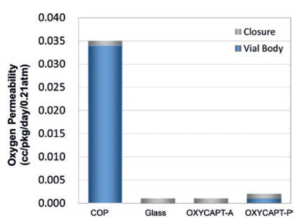
Figure 4: Oxygen barrier of OXYCAPT.
There are two types of OXYCAPT multilayer plastic vial and syringe – OXYCAPT-A and OXYCAPT-P. OXYCAPT-A has achieved a glass-like oxygen barrier (Figure 4). According to some internal studies, OXYCAPT-A can maintain a lower oxygen concentration in the headspace than Type 1 glass, thanks to its oxygen-absorbing function.
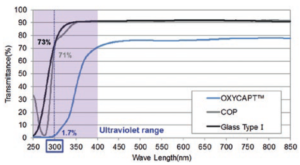
Figure 5: Ultraviolet barrier of OXYCAPT.
Although there is no oxygen-absorbing function, OXYCAPT-P has also achieved an excellent oxygen barrier. For example, the oxygen barrier of the OXYCAPT-P vial is about 20 times better than that of a COP monolayer vial. OXYCAPT-A is particularly suitable for oxygen-sensitive drugs and OXYCAPT-P is recommended for other less oxygen sensitive drugs.
OXYCAPT also has a UV barrier. For example, although about 70% of UV light of 300 nm transmits through glass and COP, only 1.7% of UV light transmits through OXYCAPT (Figure 5). We have confirmed this feature also contributes to the stability of biologics.
| Type | Volume | ISO | Parts | Option |
| Vial | 2 mL | ISO 8362-1 | Vial | Bulk or RTU |
| 6 mL | ISO 8362-1 | Vial | Bulk or RTU | |
| 10 mL | ISO 8362-1 | Vial | Bulk or RTU | |
| 20 mL | ISO 8362-1 | Vial | Bulk or RTU | |
| Syringe | 1 mL “long” | ISO 11040-6 | Barrel, Tip Cap, Stopper, Plunger Rod |
RTU |
| 2.25 mL | ISO 11040-6 | Barrel, Tip Cap, Stopper, Plunger Rod |
RTU |
Table 1: Product portfolio.
As for the product portfolio, there are four volumes for the OXYCAPT vial and two volumes for the OXYCAPT syringe (see Table 1). All the dimensions of the OXYCAPT vial and syringe are designed in accordance with the ISO standard. We can offer bulk vials, ready-to-use (RTU) vials and RTU syringes. The RTU vials and syringes are placed in ISO-based nest and tub formats and packed with a Tyvek® lid, a Tyvek bag and a high gas-barrier bag (Figures 6 & 7). All the RTU containers are sterilised by gamma.

Figure 6: Nest and tub for RTU vials. Figure 7: Nest and tub for RTU syringes.
Recently, we measured the amount of silicone oil leached from the OXYCAPT syringe and the existing Type 1 glass syringe. Each syringe was filled with distilled water and then shaken at 100 rpm for one week at 30°C. A week later, the quantity of leached silicone oil from each syringe was measured by proton nuclear magnetic resonance imaging (1H NMR) The results showed the amount of silicone oil leached from the OXYCAPT syringe was about seven times less than that from the Type 1 glass syringe (Figure 8).
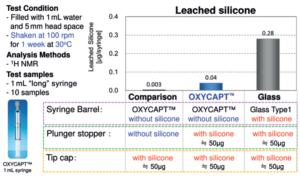
Figure 8: Silicone oil from Type 1 glass syringe and OXYCAPT syringe.
Container closure integrity (CCI) is one of the important requirements for prefilled syringes. Although dye ingress testing is popular at present, other quantitative analysis such as helium gas testing is also now conducted. In addition to dye ingress testing, we have conducted helium gas testing to confirm the CCI of the OXYCAPT syringe. Results from helium testing showed that both the OXYCAPT 1 mL and 2.25 mL syringes meet the required CCI criteria (Table 2).
| OXYCAPT S1 | OXYCAPT S2.25 | |
| Syringe Size | 1 mL long | 2.25 mL |
| Stopper | PTFE laminated stopper | PTFE laminated stopper |
| Sample Quantity | 10 | |
| Acceptance Criterion for Helium CCI |
<1 x 10-7[Pa m3/s] | |
| Leak Rate between Stopper and Barrel |
< 1 x 10-8[Pa m3/s] | < 1 x 10-8[Pa m3/s] |
| Leak Rate between Tip Cap and Nozzle |
< 1 x 10-8[Pa m3/s] | < 1 x 10-8[Pa m3/s] |
| Compliance with Helium CCI acceptance criterion? |
YES | YES |
Table 2: Container closure integrity test results.
As we have been asked to develop staked-needle multilayer plastic syringes (Figure 9) by some customers, we started tackling the development a few years ago.
We have recently decided to invest in a facility for the staked-needle syringe. The necessary equipment will be installed during 2020. The OXYCAPT syringe with a needle has some special features – it is tungsten free, glue free and adhesive free, and several gauges and lengths will be available.
Figure 9: Staked-needle syringe (under development).
CONCLUSION
OXYCAPT has been developed to overcome the current problems the pharmaceutical industry is experiencing with syringes and vials made from traditional materials. In addition to special features of COP – such as a high water vapour barrier, high break resistance,very low extractables and low protein adsorption – OXYCAPT can offer a high oxygen and UV barrier. Also, we have conducted extensive testing and developed innovative products based on customers’ requests. We believe OXYCAPT offers numerous substantial benefits to the rapidly growing pharma industry.

