Citation: Duband S, Tunkel M, “Leveraging the Patient Journey to Optimise Device Use at Home”. ONdrugDelivery Magazine, Issue 105 (Feb 2020), pp 30-34.
Séverine Duband and Mark Tunkel discuss the importance of device optimisation to increase patient safety during self-administration of medication.
Self-administration at home is becoming increasingly common for parenteral drugs, leading to needlestick injuries becoming a growing patient safety concern.
“More than three million needlestick injuries are reported every year globally.”
The growing prevalence of chronic diseases, along with the evolution of patients’ lifestyles, is driving new ways of administrating parenteral drugs. Novel treatments have become available, with the majority arising from biological molecules. Biologic drugs are predominantly administered via injectable devices, using prefilled syringes as a base.
In addition, pharmaceutical companies are striving to move injectable drugs from intravenous (IV) to subcutaneous (SC), hence avoiding hospital time for patients who largely prefer self-administration at home, instead of long and cumbersome hospitalisation. Home administration brings many benefits – lowering the cost of treatment for all stakeholders and increasing adherence to treatment regimens.
However, more than three million needlestick injuries are reported every year globally, according to the WHO – resulting in serious health, psychological and cost challenges. To boost protection for both healthcare professionals and patients, global regulations are progressively enforcing the use of safety systems for injectable drugs:
- In 2000, the US FDA issued the Needlestick Safety and Prevention Act –and the US was the first country to adopt and actively enforce legislation requiring healthcare facilities to use safety syringes
- In the EU, the new Medical Device Regulation 2017/745 (MDR) becomes mandatory this year, clearly stating as a key principle that protection of users and patients when injecting a medication with a syringe-based device is a must.
Self-administration at home therefore translates into a need for safer, easy-to-use and ergonomic devices. Having patients (or caregivers who are not healthcare professionals) injecting a drug means increased risks of use errors and needlestick injuries. Protecting users from sharps injuries, whilst optimising the injection experience, has become a must.
UNDERSTANDING THE PATIENT JOURNEY TO PROVIDE BETTER OUTCOMES

Figure 1: Applied ethnography can help designers learn about the context of use in self-administration through observations and interviews to get a broad understanding of the patient journey.
At the earliest stages of establishing the requirements and user needs for a delivery platform, Nemera works with customers to fully understand the patient journey by using a technique called applied ethnography (Figure 1). This method relies on a combination of interviews and in-context observations of practices, processes and experiences within the patient’s home or natural environment.
At this stage, potential use cases are looked at broadly – from when a patient receives their device, through the entire process of preparing, administering and disposing of that particular device. This gives the most natural view of the patient experience in use and in context. It is equally important to gain an understanding of the experience of healthcare professionals, as well as conducting applied ethnography in relevant settings in acute care or clinical environments in cases where patients may be treated in a clinic and also self-injecting at home. This is critical in helping with device selection and training for a specific patient population.
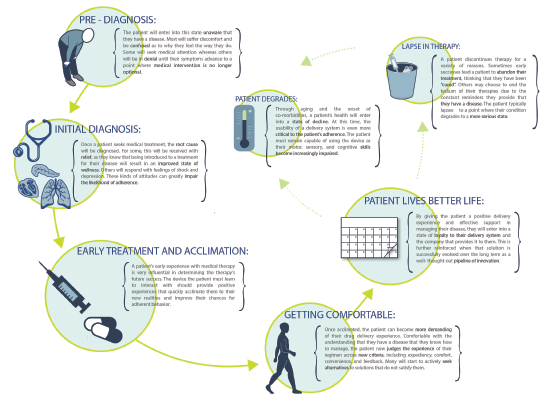
Figure 2: The output from applied ethnography is a patient or clinical journey map that allows the development team to identify opportunities for improving the user experience or mitigating risk.
Understanding both the patient and healthcare provider experience enables development of these patient journey and clinical environment process maps (Figures 2 & 3), which demonstrate the complete process patients go through in managing their disease – both from an administration standpoint and from a longitudinal perspective – as they progress with their condition and treatment.

Figure 3: An intimate understanding of the patient journey can be leveraged to identify opportunities for innovation across all stages of the patient experience.
This can be of critical importance in applications where care is being migrated from a clinical environment such as an oncology ward – with built-in support systems – to an environment of self-administration where clinical personnel are not present and the burden of support falls to a family member or caregiver. These cases are often driven by a migration in drug-delivery modality such as from IV to self-administered SC that need to be considered in a programme. The ability to gain a significant understanding of the environment, social/emotional contexts and all the other factors that influence a patient’s use of a self-injection device can be incredibly powerful in addressing these challenges.
Thoroughly understanding and mapping the patient journey and inter-relationship with healthcare provider processes can then be leveraged as an early-definition road map to help:
- Determine where customisation of a device may be warranted to address user needs further with certain patient populations
- Identify experiential gaps at an early stage, which can then be addressed through the development of instructions for use (IFU), novel training methods such as resettable trainers, value-added packaging or other methods to support clinical work as well as drive commercial differentiation
- Uncover patient engagement and adherence opportunities that can be supported with connectivity and other methods to support value-based care, which is increasingly a focus of payers and providers in the US in particular
- Identify potential areas of user-based risk that may be present in clinical trials and in market so they can be mitigated as early as possible in development.
A NEW GENERATION OF PASSIVE ADD-ON SAFETY DEVICES
Regulation and recommendations are evolving to improve patient and health worker safety. In this context, Nemera’s Safe’n’Sound product range matches the need for safe and easy-to-use self-deliveries – this passive safety device for prefilled syringes provides increased safety for patients and healthcare professionals.
Biologics have become a significant driver for new treatments, with more than 2,700 remedies in development as of mid-2017 – three times higher than in 2013.1 These new biologic drugs, such as monoclonal antibodies, are good candidates for the treatment of the aforementioned chronic diseases.
Biologic drugs are very often needed in high concentration. This can be driven by the nature of the molecule, composition of the final drug and the effort to decrease the frequency of treatment for the patient. Their viscosity sees a power law increase as the antibody concentration rises. Thus, to be injected, these molecules need to be diluted, which leads to higher injection volumes and lower (but still high) viscosity. As a result, larger-dose drug deliveries are a growing segment, with volume shifting towards 2 mL.
The Safe’n’Sound needle safety device platform is now available to accommodate 2.25 mL fill volume prefilled syringes. This new 2.25 mL size (Figure 4) is specifically relevant to administer complex, high-value drugs such as monoclonal antibodies or other biological therapies. It offers a reliable and intuitive design for user safety, and is compatible with any type of ISO standard 2.25 mL prefilled glass syringe, including various flange types.
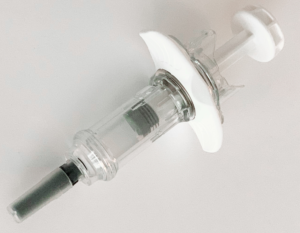
Figure 4: Nemera’s Safe’n’Sound add-on safety device is now available for 2.25 mL prefilled syringes with many customisation options.
The platform is suitable for low fill volumes and high-viscosity formulations. It is robust against shocks and vibrations, and can run with manual and fully automated assembly lines. Safe’n’Sound is a passive, one-handed safety device and is intended to be used by non-experienced users for self-administration as well as healthcare professionals.
With its ergonomic benefits, it helps patients throughout the injection without compromising on safety or robustness. Activation of needle shielding is easy and intuitive, it has a large thumb pad and extended finger flanges to optimise the injection experience, and the clear, visible tip enables easy drug inspection.
The Safe’n’Sound product range has been fully validated through user studies and is commercially available. In addition, in order to answer patients’ needs in self-administration settings, Nemera offers a fully customisable platform. For example, for patients with conditions resulting in disabilities or dexterity issues, Nemera can offer “soft touch” material to improve gripping, as well as an overcap to ease rigid needle shield removal. Colour customisation for a safer drug or dose identification is also available.
DEVELOPMENT OF SAFE, EFFECTIVE AND DIFFERENTIATED COMBINATION PRODUCTS
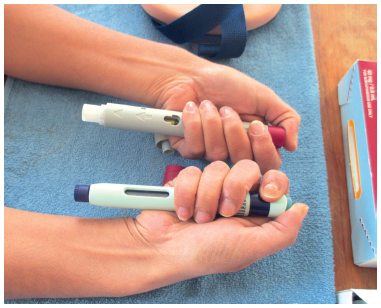
Figure 5: To support human factors submissions, evaluate the impact of innovative packaging, instructions for use, etc and differentiate with a platform device,
it is necessary to work with patients.
Using this foundation, Nemera works with customers to provide technical support, human factors and patient experience activities (Figure 5) necessary for a successful drug-device combination product development process. Customers often assume that all the human factors, risk management and characterisation of performance activities relative to their specific drug and patient attributes have already been completed as part of the platform device development.
However, this is not the case as, ultimately, pharma companies are responsible for their own drug-device combination and the platform device at that stage is really only half the picture. It’s incumbent on the pharma company to ensure their selected device, in combination with their drug, is appropriate, safe and effective for the target population. This also extends to executing the earlier stage road map to optimise the patient experience to create competitive differentiation and to ensure adherence and engagement with patients and clinical stakeholders. This may be of particular interest to customers in the biosimilars or generics markets, where many competitors are targeting the same reference drug or devices, and differentiation wherever possible is critical.
Through capabilities in design research, human factors engineering, user experience design, engineering, lab services and regulatory support, Nemera is positioned to offer all the support customers require through an integrated device platform and service programme that includes:
- Lab and analytical services to support characterisation and compatibility of drug products, syringe candidates and devices such as Safe’n’Sound to optimise their integration
- Leveraging the Insight Innovation Center’s consulting expertise to help define user groups/populations and early use-related risk analysis activities to define the human factors and usability programme necessary for the client’s regulatory/filing strategy and identified clinical risks
- Developing instructions for use, value-added packaging, connectivity add-ons to support patient engagement/adherence, and integration of resettable training devices into the patient services model to create commercial differentiation
- Conducting formative and summative usability testing globally for all aspects of the device and supporting assets in alignment with the human factors programme definition through human factors engineering report documentation for use in regulatory submissions
- Programme management excellence to ensure all elements of the programme are integrated to drive efficiency and proactively mitigate any programme risks.
This capability, combined with Nemera’s device platforms and manufacturing expertise, helps customers achieve the outcome of a successful regulatory submission and commercial launch of safe, effective and differentiated combination products while mitigating the risks associated with multiple partners who may not have expertise and experience in all the aspects critical for success.
CONCLUSION
Thanks to the increased early-stage development capabilities offered by the recent integration of newly acquired capabilities with the Insight Innovation Center, the Nemera teams in Europe and the US can now be a single partner for device platforms and integrated services – from front-end innovation, design research, human factors and design engineering to strong l ate-stage development, as well as clinical and commercial manufacturing capabilities.
These enhanced capabilities help in gaining a full understanding of the patient journey that can be leveraged to support user-experience-driven optimisations of the injection experience, to create competitive differentiation and increased patient engagement. This also extends to satisfying human factors engineering requirements for a specific drug/device combination product using Safe’n’Sound, as well as other device platforms offered by Nemera. This service offering eliminates the need for customers to use multiple resources and can result in a more efficient process and speed to regulatory filing and commercial supply.
Especially in the parenteral industry – where the evolution of patients’ lifestyles has been changing the way of administrating drugs – it is very important to understand patients’ needs and to provide them with better solutions to administer their treatment. That’s why Nemera has developed Safe’n’Sound, which is fully customisable and available in 1 mL and 2.25 mL sizes. Its robust and ergonomic design helps prevent needlestick injuries, providing user-friendly protection for healthcare professionals or inexperienced users. This design optimisation is also key for pharma companies as it has an impact on treatment efficiency and drug overfilling.
REFERENCES
- Hooven M, “Opportunities and Challenges in Biologic Drug Delivery”. Am Pharm Rev, Dec 2017.

