Citation: Braun S, “Why the Consistent Challenges Surrounding MAPS are Dissolving Fast”. ONdrugDelivery, Issue 116 (Jan 2021), pp 10–14.
Sebastian Braun discusses the current state of microneedle-based transdermal drug delivery, covering available microneedle delivery methods, the US FDA’s current stance on microneedle technologies and LTS’s microarray patch (MAP) system and the possibilities it represents.
INTRODUCTION
For the past decade, innovation in microneedles has been a focus within the pharmaceutical world. Today, microneedles are seen as a viable option for the delivery of drugs such as biologicals, vaccines and difficult-to-deliver small molecules through the skin, in both immediate-release and long-acting products. The widely recognised benefits of transdermal administration in terms of pain-free delivery, convenience and patient compliance, make microneedles an ideal platform for an increasing number of therapeutic areas.
However, the journey has not been pain free. The US FDA has clearly struggled with the quality of submissions received from combination products using microneedles, specifically around stability testing, content uniformity, risk analysis, sterility validation and manufacturing.

Figure 1: LTS’s MAP system.
This article will cover the relative merits in each form of microneedle technology; review the specific requirements set out by the FDA in October 2020; present the case for the specification of dissolvable microarray patch (MAP) technology (Figure 1), which uniquely addresses the FDA’s requirements; and finally evaluate the benefits of MAP technology to each stakeholder group, including pharma partners, payers, healthcare professionals (HCPs) and patients.
The skin is an important protective barrier with an innate reactive capability (Figure 2). Its protective, inflammatory and immunological properties make it an attractive target for efficient drug delivery. It has been well documented over the years that, for various drugs, there are many advantages to intradermal delivery compared with the intramuscular and subcutaneous routes. However, the use of a traditional needle mostly requires a relatively sophisticated cold chain supply, as well as the time and effort of an HCP. It also presents issues for the patient as it is quite invasive, particularly for the large portion of patients who suffer from needle phobia.
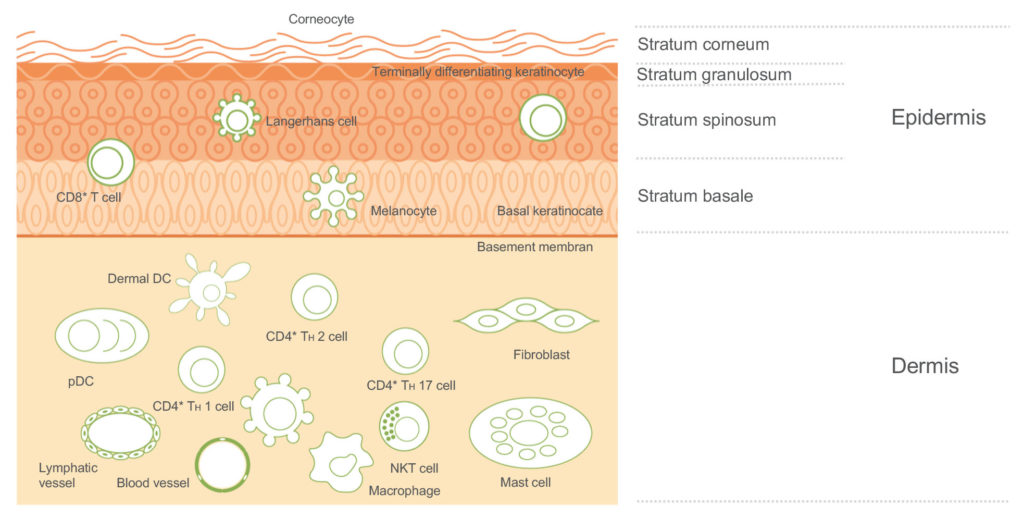
Figure 2: Skin composition.
“The pharmaceutical industry is very much aware of the benefits of drug delivery by injection in terms of its capability to deliver to the dermis, subcutaneous tissue and muscle layers under the skin. For a number of drugs, it is, in many ways, an ideal delivery route.”
As an alternative approach, MAPs can reproducibly deliver APIs into the dermal and epidermal layers of the skin, which contain high densities of immune cells.1 Microneedle technology was first conceptualised, and subsequently patented, in the 1950s,2 but it took some time for the benefits of microneedles to be fully recognised. It was not until 1998 that a paper was published exploring the possibility of using microneedles for vaccination in the future.3 Since that early work, the WHO has identified microneedles as a potential game-changer for vaccine distribution and coverage in low-to-middle-income countries (LMICs).4
NEED FOR A LESS PAINFUL WAY THROUGH THE SKIN
The pharmaceutical industry is very much aware of the benefits of drug delivery by injection in terms of its capability to deliver to the dermis, subcutaneous tissue and muscle layers under the skin. For a number of drugs, it is, in many ways, an ideal delivery route. However, there are evident issues too, specifically related to patient compliance, usability and safety for HCPs. Aversion to needles, pain and needle size are all very real concerns for many patients.
Needlestick injuries are a source of great concern for HCPs, indeed the WHO stated in its World Health Report 2002 that of 35 million HCPs, two million experience percutaneous exposure to infectious diseases each year. The WHO further noted that 37.6% of Hepatitis B, 39% of Hepatitis C and 4.4% of HIV/AIDS in HCPs globally are due to needlestick injuries.5 As well as the human cost, there is a financial cost too, with each needlestick injury case costing the local healthcare system around US$350 (£259).6 Although MAPs do not entirely eradicate the potential for needlestick injuries, they are certainly a big step in the right direction towards protecting HCPs.
THE EVOLUTION OF MICRONEEDLES
Transdermal patches offer a demonstrable improvement in patient compliance, safety and usability, and represent the delivery mechanism of choice for many therapies. The challenge is, of course, that at present the choice of API is limited to small molecules, with today less than 30 APIs having been successfully commercialised for transdermal therapeutic systems (TTSs).
These limitations have been addressed by several microneedle technologies which enable the drug to overcome the skin barrier (Figure 3). Here, the currently available transdermal microneedle options are:
- Solid removable microneedles dispense only a limited drug load and deliver a short, sustained release of drug due to the speed of the skin healing.
- Coated microneedles deliver an immediate release of API and require only a short application time on the skin. They do, however, only carry a very limited drug load.
- Hollow microneedles can carry a higher drug load and be used for immediate or sustained release, but there are limitations in the types of API solution that can be delivered this way.
- Dissolvable microneedles offer very little in terms of compromise, and a great deal in terms of benefit. Dissolvable microneedles will be returned to later in this article.
- Hydrogel-forming microneedles have the capacity to carry a higher drug load to be delivered over a sustained period. Although they present no sharp-edge waste issues, they are limited by diffusion of the API in the polymer matrix and by the number of polymers that can be used.
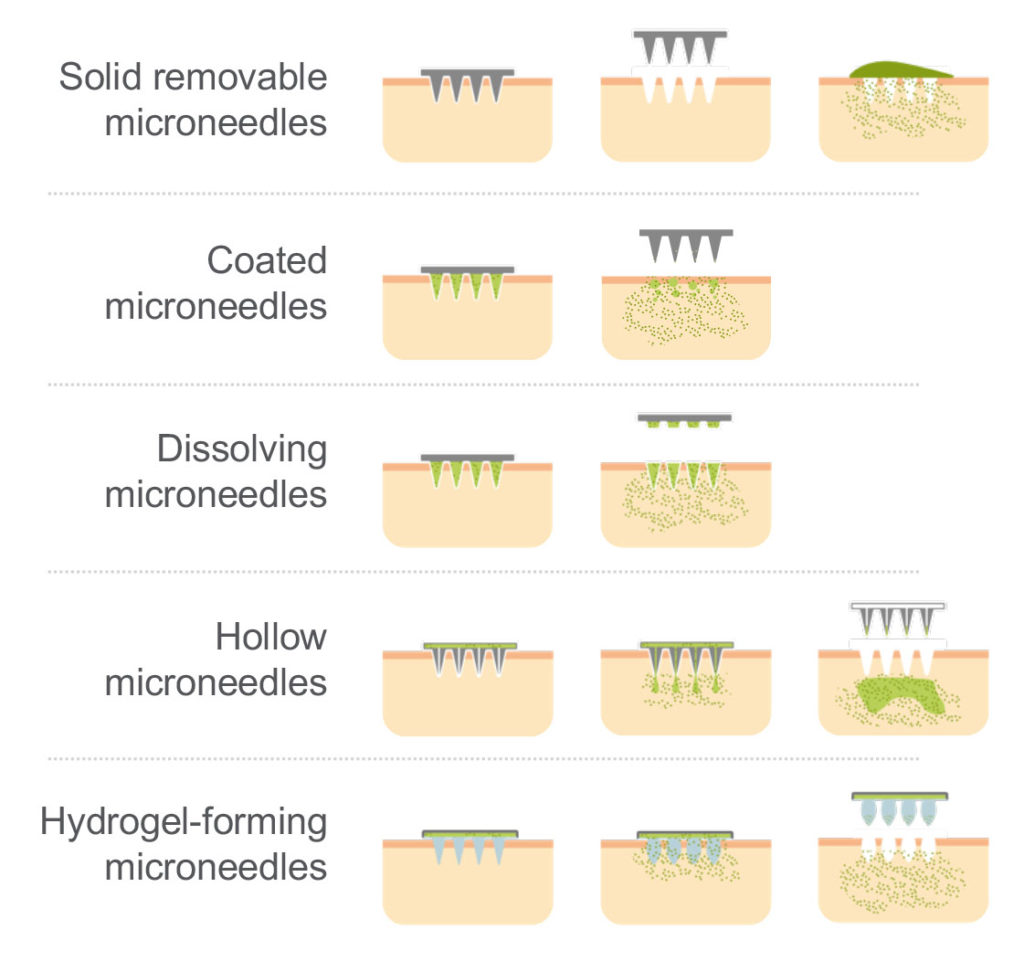
Figure 3: Overview of microneedle technologies.
All these options offer tangible benefits in terms of patient compliance and HCP safety compared with injection-based administration. It is also worth noting that solid removable, coated and hollow microneedles all result in sharps waste, whereas dissolvable and hydrogel-forming microneedles do not.
MICRONEEDLES AND THE FDA
“The requirements set out by the FDA are nothing more than would be expected for any regulated drug delivery device, and it is alarming that the quality of some submissions has been poor enough to necessitate such regulatory guidance, given the immediate and unparalleled opportunities this kind of technology presents.”
The FDA’s experience of submissions relating to microneedles to date could perhaps be described as disappointing, with quite basic failures evident in many fundamental aspects of submission. This increasing level of disappointment manifested itself in an October 2020 presentation from the FDA which sought to set out in very clear terms the criteria for a successful submission.7 In it, the FDA stated: “Regulation of combination products must take into account the safety and effectiveness questions associated with each constituent part and the product as a whole.”
The FDA’s presentation sought to clarify the common product, product-use and manufacturing deficiencies, as well as setting out what it needs to see from future applications. In terms of the common product and product-use deficiencies, the headlines related to stability testing in terms of formulation/API migration and continued testing of mechanical attributes.
Content uniformity is fundamental, and there is some work to do for delivery device designers and the pharmaceutical industry more broadly in order to establish the required specification limits and then adequately demonstrate compliance. Some submissions showed that verification and validation testing was not using the final finished combination product in bench-top, clinical or human factors studies, and there was a lack of risk analysis to ensure leaving the system in place longer than prescribed would not result in injury or overdose.
The guidance from the FDA in this regard is clear. Submissions must deliver:
- A comprehensive design control package that identifies risks and hazards
- A clearly defined control strategy for EPR, design verification and validation
- A risk management plan
- An iterative guide to risk management activities throughout the entire product lifecycle.
Common Manufacturing Deficiencies
One particular area of weakness has been incomplete facility responsibility listings on the 356h form. Process parameters and in-process controls are not supported by process development knowledge in the application. There have been significant failures with submitted batches, with no root cause analysis provided. In terms of biologics, there have been incomplete process performance qualifications (PPQs) submitted; the product not being manufactured within the review cycle and inspection times; the sterilisation validation data delivered has been insufficient; and there have been failures with equipment qualification, process simulation and sterility testing methods.
The FDA’s unequivocal requirement is for evidence of process development and the bridge to commercial scale. Submission documentation must deliver commercial process descriptions, process flow diagrams, master batch records (MBRs) and a sterilisation validation package for sterile products.
The presentation also highlighted three clear objectives for prior approval inspection (PAI):
- Readiness for commercial manufacturing
- Conformance to application
- Data integrity.
“LTS’s goal was to develop a transdermal delivery method where, if the API can be delivered via an injection, it can be delivered by LTS’s transdermal device. LTS has developed such a device with its MAP system.”
In LTS’s opinion, the requirements set out by the FDA are nothing more than would be expected for any regulated drug delivery device, and it is alarming that the quality of some submissions has been poor enough to necessitate such regulatory guidance, given the immediate and unparalleled opportunities this kind of technology presents.
Meeting FDA Guidance
For 40 years, LTS has been regarded as a trusted partner and market leader in innovative transdermal drug delivery systems. The company’s mission is to find alternatives for patients where conventional drug delivery presents challenges. As such, LTS constantly creates new technologies that support pharmaceutical development and improve patient outcomes. While LTS is clear about the efficacious benefits of TTSs, it is also aware of their limitations in terms of API fit, and so is committed to research and development towards finding a technology where the compromises inherent in current TTSs were reduced.
Essentially, LTS’s goal was to develop a transdermal delivery method where, if the API can be delivered via an injection, it can be delivered by LTS’s transdermal device. LTS has developed such a device with its MAP system. This system delivers a range of benefits to the entire healthcare ecosystem in terms of increased patient comfort and better compliance, a faster onset, lower overall healthcare costs through self-administration, lower costs per administration – via reducing the dose (‘dose sparing’) – and the removal of cold chain distribution challenges.
The LTS MAP system is based on uncoated dissolvable microneedles, which enables optimal API load without relying on liquid reservoirs. There are three key processing requirements for a homogeneous microneedle product:
- Homogeneity of dispensing solution
- Dosing precision
- Precise mould dimensions and geometry.
LTS’s MAP technology can provide for improved stability because the API is embedded homogenously in the polymer, ensuring 100% of the drug content is dispensed. The API and polymer are mixed with a solvent, dispensed into the mould and then dried to remove the solvent (Figure 4). The dispensing heads deliver a precise, reproducible volume of solution. Indeed, the dispensing heads are so precise that pharmaceutical partners can even switch formulations and volumes within each MAP. Configured for the API to be delivered and desired release option, each MAP can feature up to 1,000 needles per cm2, with needle lengths ranging from 200 μm to more than 1,000 μm – 900 μm being the threshold for pain sensation.

Figure 4: The MAP manufacturing process.
RECOMBINANT VACCINE HUMAN STUDY RESULTS
In this study, LTS aimed to investigate the safety and the general and local tolerability of the recombinant vaccine delivered by a microneedle system, as well as the efficacy of vaccination. Four cohorts were established with 12 subjects in each. Three doses of the recombinant vaccine were delivered with the LTS MAP system, according the standard ‘prime-boost-boost’ scheme. One dose level of recombinant vaccination via intramuscular (IM) injection was delivered, with boosts administered after months one and three. The results demonstrated that the recombinant vaccine MAP system is safe and well tolerated, and saw mild and moderate reactions in the subjects, comparable with those who received the vaccine via IM injection.
WHERE NEXT?
MAPs are a novel vaccine delivery methodology that has the potential to become a game-changer for immunisation programmes, especially in LMICs, which mostly rely on vaccine storage and transportation at 2–8°C and trained HCPs to administer injectable vaccines by needle and syringe. Whilst the benefits are most obvious for programmes in LMICs, that does not mean they are irrelevant for the rest of the world. Indeed, much of the development work LTS is undertaking right now is for high-value APIs.
Microneedle technology is considered an advantageous delivery route for existing vaccines, including influenza, tetanus toxoid, measles-rubella, Hepatitis B and inactivated poliomyelitis vaccine (IPV),as well as vaccines still in development, such as inactivated rotavirus and dengue. Given the challenges currently surrounding the cold-chain distribution of covid vaccines, a great deal of consideration should be given to MAPs as an efficient method to mass immunise populations.
CONCLUSION
MAPs have great potential, but it has been untapped to date. The technology has accrued advocates since the 1970s, and today there is a growing weight of evidential data to show the concerns around content uniformity have been largely eradicated. This is now a real and present opportunity for pharma partners to benefit and deliver real value across the healthcare ecosystem.
The technology overcomes the various challenges associated with conventional formulations and offers tangible benefits for pharma partners, payers, HCPs and, of course, patients:
- For pharma partners, there is now the necessary evidence to demonstrate that MAPs are a safe and reliable dosage form and that adequate patient dosing can be achieved. There are several advantages in terms of custom design too, with both sustained and immediate release options available, as well as flexibility in needle design, length, width and composition. There are also real options for patent protection and brand differentiation, as well as lifecycle management and product extension opportunities. There is also a very clear option to repurpose existing products into a more patient friendly delivery mechanism.
- For payers, there is a real benefit to MAPs in that they can enable patient self-administration, creating the potential for lower healthcare costs.
- For HCPs, they help to reduce the challenges and threats associated with needlestick injuries.
For patients, the reduced pain and lower psychological challenge of administering microneedle technologies creates the potential for increased patient compliance and adherence.
REFERENCES
- Fernando GJP et al, “Safety, tolerability, acceptability and immunogenicity of an influenza vaccine delivered to human skin by a novel high-density microprojection array patch (Nanopatch™)”. Vaccine, 2018, Vol 36(26), pp 3779–3788.
- Gerstel MS, Place VA, “Drug Delivery Device”. US Patent No 3,964,482, 1976.
- Henry S et al, “Microfabricated microneedles: a novel approach to transdermal drug delivery”. J Pharm Sci, 1998, Vol 87(8), pp 922–925.
- “WHO Microarray Patch (MAP) Product Development Workshop”. Geneva, Switzerland, 2015.
- “Needlestick injuries. Protecting health-care workers – preventing needlestick injuries”. WHO website.
- Mannocci A et al, “How Much do Needlestick Injuries Cost? A Systematic Review of the Economic Evaluations of Needlestick and Sharps Injuries Among Healthcare Personnel”. Infect Control Hosp Epidemiol, 2016, Vol 37(6), pp 635–646.
- Strasinger C, “Product Development and Quality Considerations for Transdermal Systems and Microneedle Systems: A Regulatory Perspective”. PharmaEd Resources, Microneedle Drug Delivery Systems Virtual Conference, Oct 27–28, 2020.

