Citation: Moore A, Fuensalida Pantig G R, “Creating Timeless Device Technologies”. ONdrugDelivery, Issue 125 (Oct 2021), pp 8–13.
Andrew Moore and Gene Rhode Fuensalida Pantig discuss the importance of a sound strategy for product lifecycle management.
The pandemic has led to unforeseen changes to the current disease landscape and, as a result, the increasing value of adopting either hospital- or home-based treatments can be seen. The data show telemedicine in the form of hybrid virtual/in-person care models are increasingly favoured by patients. This uptake can be seen even in chronic diseases – fields of interest in the self-injection space, such as rheumatology and endocrinology. Likewise, the injectable drug delivery market is expected to rise at a compound annual growth rate (CAGR) of 12.9% to reach US$1.251 trillion (£915 billion) by 2027. Although more of a correlation, these factors highlight the importance and urgency of home-based treatment and care, whenever possible.1,2,3
Viewing these staggering numbers in the context of the pandemic, the concepts of speed and agility have reached a whole new level of importance as we see global regulatory guidance, support and consequent approvals of biologics that are relevant to the covid-19 pandemic. From the usual time frame of 12–15 years for a molecule to reach the market, we see not only the approval but also the ramped-up manufacture and commercialisation of these products in a fraction of the time. Speed and agility are tantamount to successfully ensuring that the right product is provided to the patient at the right time. Here, we also refer to time in the context of a product staying relevant as a function of the current but ever-changing healthcare needs.4,5,6
THE CONTINUING RISE OF BIOLOGICAL PRODUCTS
While it was expected that biologics approvals would waver in the last year due to pandemic disruptions, the US regulatory landscape saw an otherwise positive turn of events. In fact, 2020 was one of the US FDA’s top three years (since 1996) when it comes to the total number of biologics approvals. Looking further at data sets from the years 2003–2018 (Figure 1), the US and EU approvals of biopharmaceuticals remained strong, and many of these are available in self-injection formats targeting various diseases. Over the years, autoinjector products have been treating rheumatoid arthritis, migraine, multiple sclerosis, type 2 diabetes, ulcerative colitis and Crohn’s disease – and recently even addressing hypercholesterolemia, as well as atopic disorders and weight management.
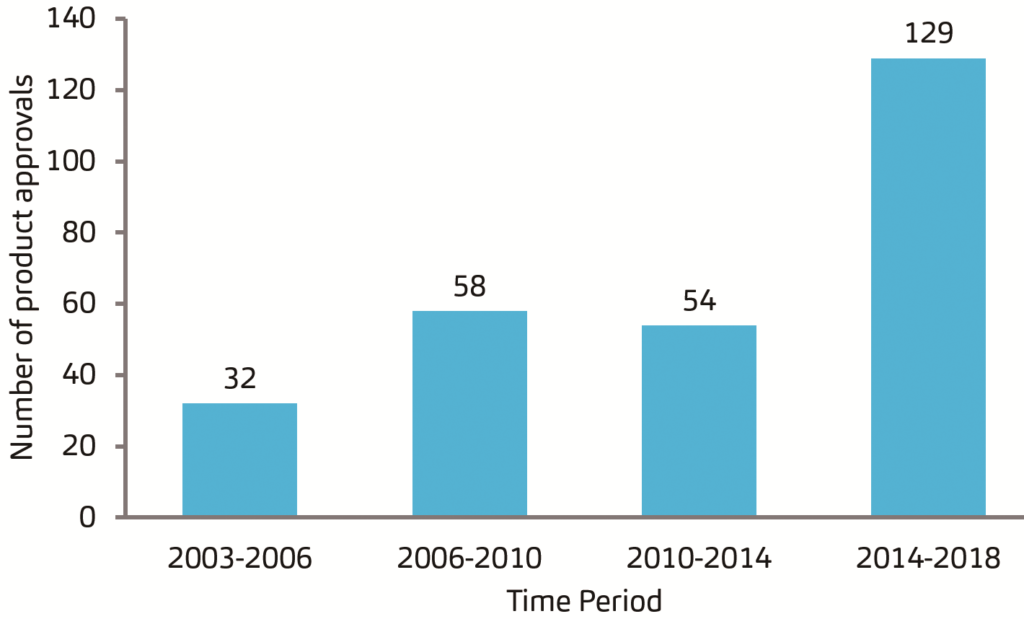
Figure 1. US and EU approvals of biopharmaceuticals over the years.
It is worth enumerating such disease areas addressed by autoinjector products, most especially when news pieces – like those published in Nature Biotechnology – strongly associate prefilled pens with patient convenience and patient centricity.7–10 With decades of experience in the drug delivery space, SHL Medical has been at the forefront of co-developing these products with pharmaceutical companies, including some of the world’s bestselling, essential medicines available in self-injection forms. SHL’s experience in combination product development has matured into a track record of designing, developing and producing the device technologies for innovator biologics – the legacy products – and their biosimilars that are developed and approved across various regions of the world.11
LEGACY PRODUCTS AT A GLANCE
A legacy product, as has been known in various industries, usually refers to an item that is no longer sold, has lost substantial market share or is a version of a product that is not current. This, however, positively implies that a legacy product may have been a blockbuster unit in the past. In some cases, a legacy product may be one that is still in current demand. While being a legacy product certainly connotes polar, contrasting qualities, it is fallacious to say that a legacy product in the autoinjector space is rendered obsolete when flexible and adaptive device technology is built around the primary container.12
In meeting the constantly changing challenges in the self-injection space, SHL’s philosophy and practice have always centred around being adaptive. For SHL, to create timeless device technologies is to create self-injection systems that are not only defined by their tangible form (core mechanism and specifications, device colour, geometry and industrial design) but also by their intangible and associated features. These attributes refer to the product offering’s ability to adopt the requirements of the drug, the customer and its patient, and adapt to the ever-changing external market drivers – effectively creating an augmented product.
For example, SHL’s second generation of Molly® that is built with a modular platform technology – and current DAI® (disposable autoinjector) products experiencing an unprecedented production ramp up – are the result of constant improvements in existing device technologies and their ecosystems. In a sense, this concept of augmentation can be closely related to product lifecycle management (PLM) in the autoinjector space.
SHL’S PLM STRATEGY
“Leveraging device market, industry and regulatory insights, as well as looking upstream of combination product development to identify emerging trends and unmet needs, SHL sees the need to take PLM to the next level.”
PLM, as generally defined in various industries, is the process of managing a product’s lifecycle from inception, through design and manufacturing, to sales, service and eventually retirement. As a modern emerging discipline in the field of product development, the first recorded application of PLM dates back to the 1980s, when American Motors Corporation used a data-driven approach to track and improve the market performance of its products from inception to end of life. In essence, PLM brought into focus the necessity to effectively manage the lifecycle of a product and render it competitive, and this is why we have seen the rise of improved manufacturing concepts such as computer-aided design and automation, as well as database management, in the drug delivery systems industry.13,14,15 Leveraging device market, industry and regulatory insights, as well as looking upstream of combination product development to identify emerging trends and unmet needs, SHL sees the need to take PLM to the next level. In 2021, SHL further fortified efforts on its PLM strategy by expanding its dedicated team of experts that lead the global product management organisation. In brief, global product management (Figure 2) is a function that was created to define and continuously develop SHL’s existing and future product and service portfolio, and facilitate product standardisation and modularisation, as well as create a tight-knit collaboration between SHL and pharma as a combination product matures over time.
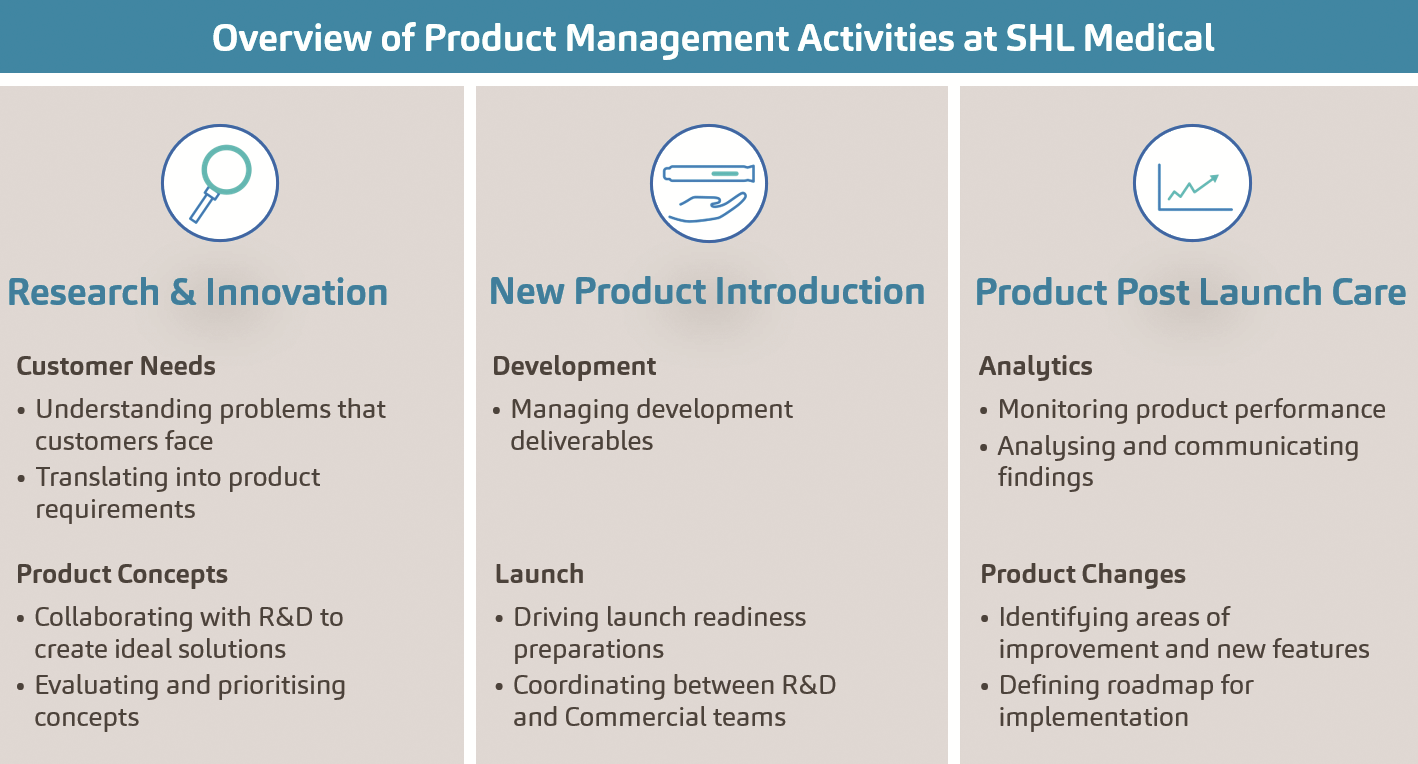
Figure 2. An overview of SHL’s product management activities, segmented according to product lifecycle phases.
In detail, SHL’s product management strategy aims to further strengthen, support and ensure the success of products co-developed with pharma partners, made possible with the following checks and balances:
• Continuously building internal knowledge of pharma’s needs by:
– Forming two-way communication channels with customers
– Identifying opportunities for new product solutions
• Monitoring product performance through:
– Operational performance
– Quality performance – Post-market surveillance
• Identifying improvement opportunities throughout a product’s lifecycle by:
– Engaging with internal and external stakeholders
– Planning implementation of changes.
As products mature, the market itself becomes highly competitive. To this end, driving growth and marketability of SHL’s device technologies and the pharma partner’s combination products become an active pursuit rather than a passive endeavour.
LIFECYCLE MANAGEMENT IN ACTION
SHL’s DAI autoinjector technology is a good example of how PLM has been proactively upheld within the organisation. Likewise, SHL’s composite experience and learnings with DAI are a great precedent for modern, up-and-coming medtech device companies wishing to ensure a robust product offering in such a highly competitive industry. First launched commercially in 2006, the DAI is one of the world’s first modern prefilled pens. The device is a button-activated, three-step autoinjector that houses 1 mL prefilled syringes, and its technical specifications certainly paved the way for how SHL’s device portfolio matured and expanded. At present, SHL has developed an array of drug delivery systems that range from being two-step to three-step devices, as well as technologies that address pharma’s biologics pipeline characterised by varying fill volume and viscosities.
As a case in point, there are two sides of the coin that we may evaluate here – DAI currently as a business-to-business device offering to pharma companies but also DAI as a legacy device technology that caters for some of pharma’s longstanding blockbuster combination products. On the first point, it could be said that DAI has certainly helped shape the industry developments on two- and three-step devices, as well as manual versus automatic needle insertion controls. The latter point merits a crucial discussion and exhibits the importance of SHL’s commitment to an active pursuit of product management with its pharma partners.16 Over approximately 15 years, the DAI technology has supported the regulatory approval and commercialisation of nearly 20 combination products. These self-injection devices, available in varying dosage presentations, are indicated for diseases such as rheumatoid arthritis, anaemia, migraine, hyperlipidaemia and osteoporosis, to name a few. Figure 3 exemplifies the depth and breadth of disease areas that the technology has addressed over time.
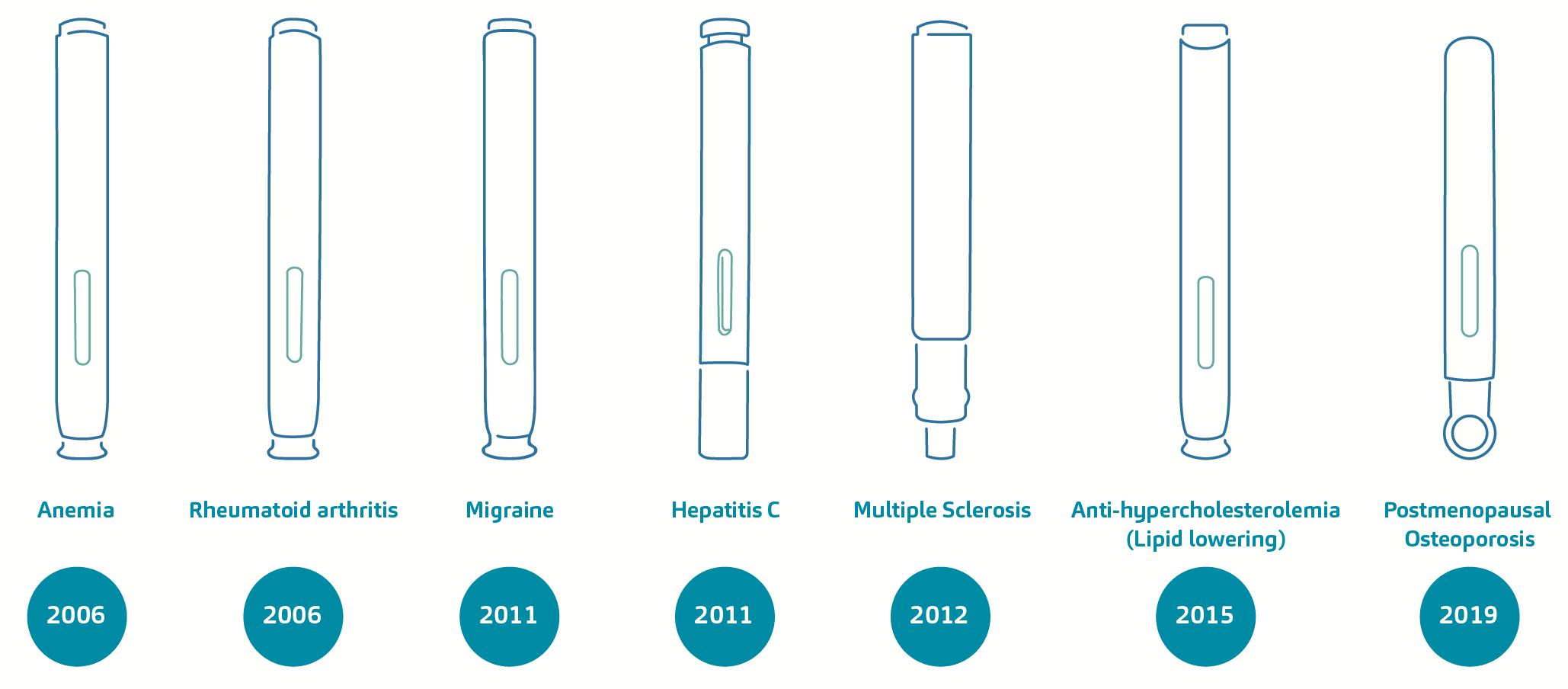
Figure 3. A non-exhaustive overview of combination products based on the DAI technology and their treatment areas. From the first DAI device, iterative developments over the years gave rise to devices inspired by the original version, and these were in the form of two- and three-step autoinjectors featuring manual or automatic needle insertion controls.
From the original device design, iterative developments over the years gave rise to devices inspired by the original version, and these were in the form of two- and three-step autoinjectors featuring manual or automatic needle insertion controls. It could be said that these early developments have influenced the progression of SHL’s device portfolio itself, and the trends within the drug delivery device industry.
A classic device that found success in its first project, SHL’s DAI is the autoinjector technology behind a blockbuster product for rheumatoid arthritis. It also supports leading combination products indicated against anaemia and migraine – diseases with a high global prevalence and which present sufferers with disease burden and disability.17
The first DAI project was developed for a multinational biopharmaceutical company headquartered in the US. The accomplishments of this partnership gave rise to succeeding device projects under the DAI technology, effectively creating a product family for SHL’s pharma partner. Given that the first project dates back to 2006, the present-day market landscape enabled SHL to evaluate upstream and downstream of the device development and production streams across the whole programme that it co-managed with the pharma partner. Applying a lifecycle evaluation approach across the board saw the need for a product portfolio consolidation; there was a need to scale up the programme.
This complex yet holistic activity was addressed through a streamlined approach to scaling up. The whole process touched upon all device designs, production and in-process controls, through to batch release testing across the programme, ensuring that complexities are minimised and process variations are reduced throughout. In brief, a design for manufacturability and assembly assessment was conducted to optimise the designs from an automated and scalable process perspective. This assessment allowed for maintaining brand recognition but also colour differentiation across the industrial designs of each device within the programme. With SHL moving towards automating many of its processes, this exercise also enabled centralisation of programme production flow. Now, the assembly process is automated across the programme, all the while incorporating historical learnings and controls.
“With more platform products emerging in the autoinjector space, SHL sought to redefine how platform device technologies can bring differentiation within the market.”
A LIFECYCLE APPROACH TO PRODUCT STANDARDISATION AND MODULARISATION
The establishment of the Molly platform exemplifies SHL’s product management and design philosophy, which is to incorporate standardisation as well as modularisation across its device technology offerings. Introduced in 2010, Molly is SHL’s first preconfigured autoinjector designed to help pharmaceutical companies reduce initial investments and expedite development timelines. With a vertically integrated development model, Molly’s platform-based infrastructures allow SHL to undertake various overlapping device projects. In 2016 alone, this preconfigured offering enabled the commercial launch of at least three combination products indicated for migraine, inflammatory and autoimmune diseases.
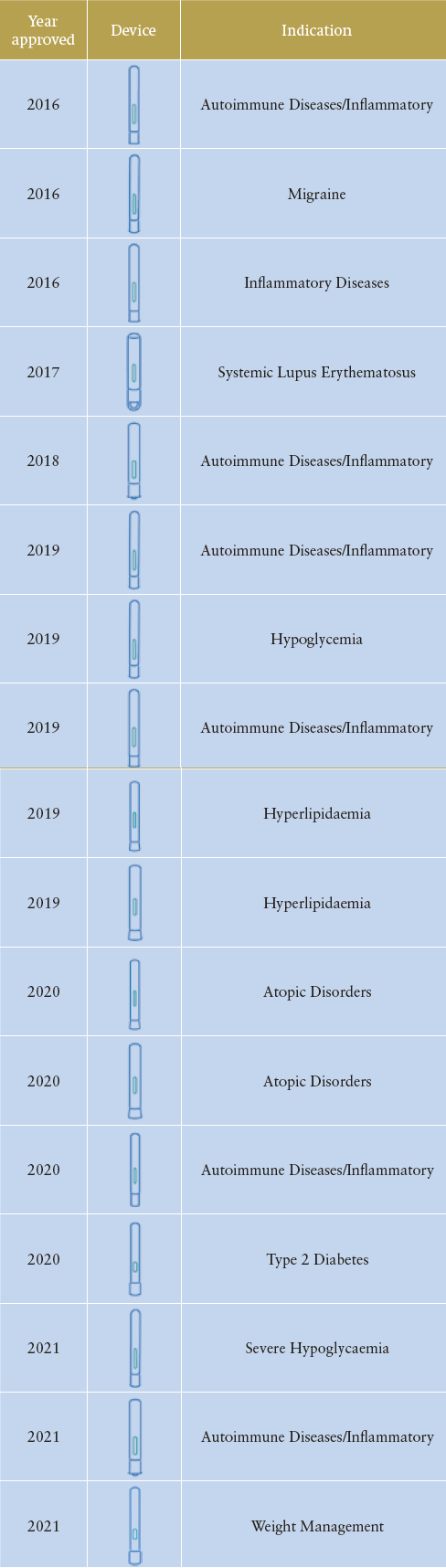
Table 1: Combination products launched over the years that
were built with the Molly autoinjector technology (brand
names have been redacted and the list of disease indications
is non-exhaustive).
Building on the successes of its predecessor, this second generation of Molly has so far resulted in customised device families for one of SHL’s leading pharma partners. Tasked to develop a device for two different biologics, the resultant combination products feature distinct industrial designs that are conformant to the primary container. Of important note, the Molly 2.25 variant supported the development and commercialisation of one of the world’s first autoinjectors in the higher volume range (≥2.0 mL). Incorporating a lifecycle approach has proven to be successful for the maturity of the Molly modular platform, and a list of its commercial successes can be seen in Table 1.
This is not to say that there may be no room for improvements for such a device technology. With more platform products emerging in the autoinjector space, SHL sought to redefine how platform device technologies can bring differentiation within the market. Further refined to offer the advantages of platform products while offering flexibility in the device’s design, development and production, the Molly modular platform was born.
“The fate of device technologies will always depend on a sound lifecycle management strategy.”
CONCLUSION
The fate of device technologies will always depend on a sound lifecycle management strategy. Consequently, the importance, value and impact of combination products to end users will largely depend on the resonating beneficial experience when these products are used over time. To this end, SHL is committed to developing device technologies that are timeless. By timeless, we mean constantly adding value within the device chain as well as futureproofing the ecosystem surrounding device technologies. This includes taking proactive measures in sustainability by constantly evaluating the carbon footprint, as well as investing in the research and development of digital medicines. Transcending beyond dated device technologies and the medtech industry notion of offering “just devices” is a constant objective of SHL.18
The present SHL Medical portfolio and the suite of infrastructures surrounding each device technology offering came to be, not instantaneously, but by a series of holistic learnings and improvements applied over the years. The company’s ultimate goal is enabling patients’ independence and, to this end, it is dedicated to advancing its offerings, ensuring that they positively disrupt the healthcare landscape and influence the positive progression of the drug delivery space.
REFERENCES
- Bestsennyy O et al, “Telehealth: A quarter-trillion-dollar post- COVID-19 reality?”. McKinsey & Company, Jul 2021.
- Peterson S et al, “Nonresponse Bias Report for the 2020 Household Pulse Survey”. United States Census Bureau, Mar 2021.
- “Injectable Drug Delivery Market to Rise at a CAGR of 12.9% by 2027 Owing to Increasing Prevalence of Chronic Diseases: Fortune Business Insights™”. Press Release, Fortune Business Insights, Apr 2021.
- Van Norman G, “Drugs, Devices, and the FDA: Part 1: An Overview of Approval Processes for Drugs”. JACC: Basic Transl Sci, 2016, Vol 1(3), pp 170–179.
- Anderson L, “FDA Drug Approval Process”. Drugs.com, Apr 13, 2020.
- Hartl A, Goldinger M, “Supporting Parenteral Product Development”. PMPS Parenteral Technology Supplement, 2021.
- Hodgson J, “Refreshing the biologic pipeline 2020”. Nat Biotechnol, 2021, Vol 39, pp 135–143.
- Walsh G, “Biopharmaceutical benchmarks 2010”. Nat Biotechnol, 2010, Vol 28(9), pp 917–924.
- Walsh G, “Biopharmaceutical benchmarks 2014”. Nat Biotechnol, 2014, Vol 32(10), pp 992–1000.
- Walsh G, “Biopharmaceutical benchmarks 2018”. Nat Biotechnol, 2018, Vol 36(12), pp 1136–1145.
- “WHO Model List of Essential Medicines, 20th List (April 2017)”. WHO, Aug 2017.
- Kuka M, “Product Lifecycle Management – Terminology and Applications”. Product Development and Management Strategies, 2018.
- “What Is Product Lifecycle Management (PLM)?”. SAP Insights.
- Segal T, “Product Lifecycle Management (PLM)”. Investopedia, Aug 2021.
- Watts S, “Data Lifecycle Management Explained”. BMC, Jun 2018.
- McGowan M, “30 Years in the Making – an Inside Perspective on the Emergence of Autoinjectors”. ONdrugDelivery Magazine, Issue 101 (Oct 2019), pp 20–23.
- “Disease incidence, prevalence and disability”. WHO, 2004.
- “SHL Sustainability”. Company Web Page, SHL Medical, Sep 2021. (www.shl-medical.com/sustainability, accessed Sep 22, 2021).

