To Issue 130
Citation: Shetty G, Whelton R, “Ophthalmic Injection of Viscous Formulations – Why Unique Needs Require a Novel Delivery Approach”. ONdrugDelivery, Issue 130 (Mar 2022), pp 54–58.
Guatam Shetty and Richard Whelton outline the key considerations related to ophthalmic injection of viscous formulations and propose an innovative, user-validated and fully mechanical device-based solution.
“The main drivers behind this trend are similar to those observed in parenteral delivery, including more long-acting drugs, high-strength formulations and bi-specific antibody drugs, as well as the need to store injectables at a low temperature.”
THE TREND TOWARDS VISCOSITY
The trend towards more viscous formulations is widely acknowledged in parenteral drug delivery. The same trend is now also occurring in ophthalmic drug delivery, which includes formulations for intravitreal, subretinal and intracameral injections. However, given some of the unique attributes of the eye, the solutions to drug delivery challenges presented by high-viscosity ophthalmic formulations will be different from those for parenteral formulations.
The introduction of anti-vascular endothelial growth factor (anti-VEGF) agents administered via intravitreal injections transformed the treatment of retinal disorders such as age-related macular degeneration (AMD), diabetic macula oedema (DMO), diabetic retinopathy (DR), retinopathy or prematurity (ROP) and branch and central retinal vein occlusions (BRVO and CRVO). However, many challenges in ophthalmology remain to be solved and the occurrence of retinal disorders continues to increase, driven by factors such as an ageing population and the rising prevalence of diabetes.
Pharmaceutical investment in the ophthalmology sector is growing, with many injectable formulations in the pipeline. Within the injectable pipeline, an increasing number of viscous formulations (>20 cP at room temperature) are anticipated. The main drivers behind this trend are similar to those observed in parenteral delivery, including more long-acting drugs, high-strength formulations and bi-specific antibody drugs, as well as the need to store injectables at a low temperature.
Long-acting drugs are a significant driving factor – one to which the story of anti-VEGF agents provides good context. Historically, anti-VEGF agents have typically been administered every4–6 weeks, and there is a strong desire to reduce this frequent injection schedule. The high frequency of injections has become a burden on the healthcare system as the patient population continues to grow; furthermore, non-compliance by patients is a concern, as it can cause sub-optimal clinical outcomes. Long-acting drugs can address these challenges.
Genentech recently received approval for the Susvimo™ implant, a port delivery system for slow, controlled release of ranibizumab for the treatment of wet AMD. A related approach is high-strength dosing, with an in-pipeline example being a high-strength dose of Eylea® (aflibercept – Regeneron). High-strength formulations tend to be more viscous, with some drugs in the pipeline being extremely so. Bi-specific antibodies are another class of therapeutics known for high viscosity. Several of them are in the pipeline for ocular delivery, such as the recently approved Vabysmo™ (faricimab-svoa – Genentech).
The requirement for these and other molecules to be stored at low temperatures is also an important factor to consider. Viscosity increases with lower temperature, and clinicians often do not wait until a drug reaches room temperature before injecting. This occurs for several reasons but always results in an amplification of the drug’s viscosity, for already highly viscous formulations.
VISCOSITY-RELATED CONSIDERATIONS FOR OPHTHALMOLOGY
The need to generate an increased injection force is a known challenge when injecting high-viscosity drugs. The Hagen-Poiseuille equation can help estimate the injection force required during drug delivery. It shows how greater viscosity requires a higher injection force, as well as how other parameters impact injection force. By analysing each variable, it is possible to explore options for reducing injection force when injecting viscous drugs into the eye.
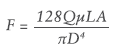
F – injection force
Q – volumetric flow rate
μ – dynamic viscosity
L – needle length
D – needle internal diameter
A – syringe internal cross section
Viscosity
In parenteral drug delivery, one of the key approaches for handling viscous drugs is to reduce the viscosity by diluting the formulation. However, this increases the injection volume, which could cause a harmful increase in intraocular pressure if injected into the relatively small, fixed volume of the eye. Therefore, this approach is simply not a practical option for ophthalmic delivery.
The dependence of viscosity on temperature is an important, related factor. As mentioned previously, retina specialists often administer ophthalmic drugs without waiting for them to reach room temperature first, thereby increasing the likelihood that the drug is injected at an effectively higher viscosity.
Needle Internal Diameter
The Hagen-Poiseuille equation also shows us that a needle with a larger internal diameter reduces the injection force required, and using a larger needle is often a possibility for parenteral drug delivery. However, for injections into the eye, a thin needle is necessary to avoid complications; for example, larger needles can cause an intraocular pressure spike due to their higher penetration force. As such, ophthalmic injections are already predisposed to have higher injection forces, with viscous formulations further compounding the situation.
Volumetric Flow Rate Reducing flow rate can help reduce the injection force. However, lower flow rate would mean longer injection time and therefore a longer residence time of the needle in the eye. This, combined with higher injection force for viscous formulations, could lead to instability and potential injury to the patient. Also, in the case of intravitreal injections, the patient is awake and aware of the fact that a needle is in their eye. Longer injection times could be a significant source of distress for patients and therefore deter compliance with their treatment. Injection times longer than 2–3 seconds would be considered a deviation from current clinical practice for intravitreal injections.
Syringe Internal Cross Section
The Hagen-Poiseuille equation indicates that using a syringe with a smaller cross section can help reduce injection force. For example, a 0.5 mL standard prefilled syringe (PFS) will have a lower injection force than a 1 mL long PFS. This is not a common strategy in parenteral delivery, as injection volumes tend to be 1 mL or greater, but is a potential option for ophthalmic drug delivery. The 0.5 mL PFS represents a possible reduction of syringe internal diameter in a standard size.
Needle Length
Decreasing the needle length can reduce the injection force. For intravitreal injections, standard practice is to use a 13 mm needle, which enables the needle tip to be placed in the vitreous cavity. Use of a shorter needle to reduce injection force must also ensure that the needle tip is able to reach the intended injection site.
“The most logical option to consider is a drug delivery device with a mechanical advantage, such as the Congruence Microlitre Dosing Syringe.”
NON-VISCOSITY CONSIDERATIONS FOR OPHTHALMIC DELIVERY
Manual User Control
In parenteral drug delivery, especially for self-injection, one approach to produce high injection forces is the use of a stored energy source, such as a spring or compressed gas. However, in ophthalmology this would run counter to retina specialists’ preference to have complete control of the injection procedure, including injection of the drug itself. Therefore, stored energy is not a viable option.
Ensuring Sterility
In ophthalmic injections there is a risk of infection – endophthalmitis – that can lead to major complications, including blindness and possibly even death. As such, statutory requirements mandate that ophthalmic drug delivery devices, including PFSs, must be terminally sterilised. This requirement can be particularly challenging for electromechanical drug delivery systems, which are likely to be reusable for cost purposes. Liability and the burden of sterilising reusable delivery devices would be unacceptable for healthcare facilities, so single-use delivery solutions are preferable. Also, some ophthalmic injections are conducted in an operating room, so electromechanical systems would also need to demonstrate that they would not interfere with other operating room equipment.
Small, Low-weight Profile
Stability during the ophthalmic injection procedure is very important. The pars plana route for intravitreal injections ensures that the injection needle avoids impacting the eye lens or perforating/detaching the retina; injection devices would be considered too bulky if they move the centre of gravity for the device too far away from the needle insertion site, thereby causing potential instability during the drug injection step. Again, this could be a disadvantage for electromechanical devices.
INJECTING A VISCOUS FORMULATION INTO THE EYE
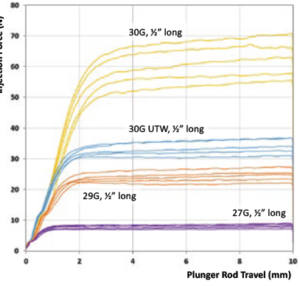
Figure 1: Comparison of injection force for various needles.
Congruence Medical Solutions has conducted numerous studies in the ophthalmic space. Based on user feedback, the target injection force for manual intraocular injections should ideally be below 20 N, which is comparable to an acceptable injection force in parenteral drug delivery. Congruence’s data indicates that there are three ways to potentially reduce the injection force to this level with conventional injection devices, although due to the various factors outlined previously, this may well not be sufficient or appropriate.
1 – Select the Right Injection Needle
A larger needle leads to a lower injection force and vice versa. Figure 1 shows injection forces when injecting a 100 cP formulation from a 1 mL long Gerresheimer tamper evident Luer lock (TELC®) syringe with baked-on silicone using different needle gauge sizes (all 13 mm long). The experience of using a 27G needle in a PFS for Macugen (pegaptanib – Bausch+Lomb) was not well received by retina specialists; the most common intravitreal injection needle size is 30G. Assuming that the extra-thin-wall 30G needle has sufficient column strength, it can be a good choice for reducing injection force. Reducing needle length could also assist in reducing injection force as long as the drug is still injected into the target site.
2 – Increase Injection Time
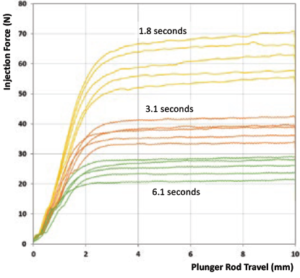
Figure 2: Comparison of injection force for various injection times.
For a 50 μL dose, Figure 2 shows injection force corresponding to various injection times for a 100 cP formulation injected using a 1 mL long Gerresheimer TELC syringe with baked-on silicone using 30G 13 mm needle. Even with a six-second injection time, the injection force would still be considered unacceptably high; patients are alert during an intravitreal injection, so retina specialists prefer for the needle to be in and out of the eye as quickly as possible. Longer injection times could, however, be considered for subretinal injections.
3 – Use a Syringe with a Smaller Internal Diameter
Congruence’s studies show that a 0.5 mL PFS has lower injection force than a 1 mL long syringe, as shown in Figure 3. However, there is no standard PFS size that has a smaller internal diameter than a 0.5 mL PFS.
Figure 3: Comparison of injection force for 0.5 mL PFS and 1 mL long PFS.
AN ALTERNATIVE APPROACH
It is very possible that all three injection force reduction strategies, even when used in combination, are inadequate for administering a viscous formulation to the eye. In this case, the most logical option to consider is a drug delivery device with a mechanical advantage, such as the Congruence Microlitre Dosing Syringe (MDS). The mechanical advantage designed into the device enables it to deliver a drug formulation at a high injection force but with the user experiencing a much lower force. The device works with standard PFSs, is manually operated, provides accurate microlitre dose delivery and is disposable and compact, thus meeting the criteria previously outlined.
The performance of this device strategy, combined with the aforementioned injection force reduction strategies, is illustrated in Figure 4. This graph shows the results from a benchtop study that measured injection forces for a 100 cP formulation when using a 1 mL long Gerresheimer TELC syringe with baked-on silicone using a 30G 13 mm needle. Figure 4 shows a comparison of injection forces with and without the Congruence MDS.
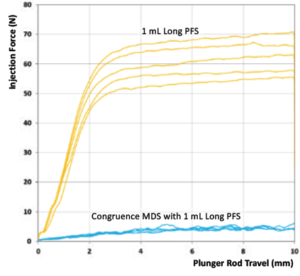
Figure 4: Comparison of injection force without and with Congruence MDS.
The results are clear – for the same plunger rod speed, there is a distinct reduction of the injection force felt by the user when using the Congruence MDS. In other words, the device generates a high injection force on the drug but the user should feel forces more akin to a regular injection, meaning that it remains easy to use and control.
USER STUDY TO CONFIRM CONGRUENCE MDS BENEFIT
In order to validate this benefit of the Congruence MDS, a user study was conducted with retina specialists. 20 retina specialists provided informed consent to participate in the study. The users were asked to inject two different formulations – one of 100 cP and one of greater than 300 cP. The injections were performed using a 1 mL long Gerresheimer TELC PFS with baked-on silicone using a 29G 13 mm needle – comparison was made between the same PFS and needle with and without the Congruence MDS. In each instance, the users were asked to quantify their acceptance of the force they had to apply in order to inject on a sixpoint scale – Completely Acceptable, Acceptable, Somewhat Acceptable, Somewhat Unacceptable, Unacceptable and Completely Unacceptable.
The results are summarised in Figure 5 and clearly show a near-universal acceptance of the Congruence MDS for both the formulations tested. In comparison, the PFS-only configuration was only acceptable to one of the users for the 100 cP injection and to none of the users for the 300 cP injection. Therefore, it is also no surprise that the Congruence device was highly preferred by participants. Other key highlights from this user study on concerns with the PFS-only configuration were:
- One user indicated that they might only be willing to inject one 100 cP formulation per day with the PFS-only configuration due to the effort required.
- Several users raised patient safety concerns from the lack of stability experienced when injecting with the PFS-only configuration – for example, potential of the needle impacting the lens.
- Users who did manage to inject the 100 cP with the PFS-only configuration expressed concern about the length of time required to perform the injection.
- Most users could not even complete the 100 cP injection when injecting with the PFS-only configuration and none could with the >300 cP formulation.
- Injection force with the PFS-only configuration was so high that 26.3% of the users could not distinguish between the 100 cP and >300 cP formulations during the test.
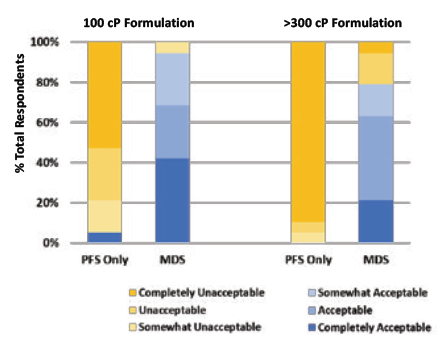
Figure 5: Comparison of injection force of PFSs without and with Congruence MDS.
SUMMARY
The approaches (with supporting data published) for injecting viscous formulations parenterally are varied and, thus far, there is no consensus solution to the challenges of high viscosity. Even then, the unique attributes of the eye mean that many of the potential solutions are not suitable for intravitreal delivery. However, a compact, mechanical, sterilisable device, such as the Congruence MDS, may enable comfortable injection of a viscous formulation into the eye using a fine-gauge needle. This requirement would be in addition to being able to deliver an accurate, precise microlitre volume dose.
The authors would like to acknowledge the contributions of Destry Rochester Jr (Lab Manager), Andrew Schaefer (Senior Product Development Engineer) and Ashwan Lewis (Quality Manager), all of Congruence Medical Solutions, and Lance Wetzel, formerly Program Manager at Congruence Medical Solutions, in generating the data reported here.
BIBLIOGRAPHY
- Tilegenova C et al, “Dissecting the molecular basis of high viscosity of monospecific and bispecific IgG antibodies”. MAbs, 2020, Vol 12(1), Article 1692764.
- Harald D et al, “Bispecific Anti-Vegf/Anti-Ang-2 Antibodies and their Use in the Treatment of Ocular Vascular Diseases”. International Patent WO/2014/009465.
- Stern ME, Schaumburg C, “IL23p19 Antibody Inhibitor for Treating Ocular and Other Conditions”. International Patent WO/2011/159655A2.
- Regula JT et al, “Targeting Key Angiogenic Pathways with a Bispecific Crossmab Optimized for Neovascular Eye Diseases”. EMBO Mol Med, Nov 2016, Vol 8(11), pp 1265–1288.
- Watt RP, Khatri H, Dibble ARG, “Injectability as a Function of Viscosity and Dosing Materials for Subcutaneous Administration”. Int J Pharm, Jan 2019, Vol 554, pp 376–386.
- Kozak I et al, “Prefilled Syringe Needles Versus Standard Removable Needles for Intravitreous Injection”. Retina, 2006, Vol 26(6), pp 679–683.
Previous article
KEEPING UP WITH THE DEMAND FOR EFFECTIVE OPHTHALMIC DRUGSNext article
ENVISIONING NEW HORIZONS IN OCULAR DRUG DELIVERY
