To Issue 138
Citation: Whelton R, Green P, “Autoinjectors: Historical Achievements and Compelling Needs Driving Next-Generation Devices”. ONdrugDelivery, Issue 138 (Oct 2022), pp 14–17.
Richard Whelton and Philip Green summarise the history of autoinjectors, highlight success stories to date, review the unmet needs that remain and consider emerging needs that could drive continued innovation in autoinjectors to ensure safety, patient satisfaction and pharma commercial success.
“Autoinjectors themselves have evolved significantly to address multiple and changing self-injection needs.”
The needle-based autoinjector market has grown significantly since the first commercial release in the 1980s and is now one of the most important modes of drug delivery. In 2021, six of the 30 top-selling drugs worldwide were delivered in an autoinjector format, representing around 50% of the total sales. The growth of the autoinjector market is expected to continue, with a projected 18% compound annual growth rate from 2019 to 2027.1
Autoinjectors themselves have evolved significantly to address multiple and changing self-injection needs. However, several unsolved problems remain and, as the pharma market itself continues to evolve, additional compelling unmet needs continue to arise.
HISTORICAL PERSPECTIVE
Enabling Self-Injection Through Ease of Use and Safety
The needle-based autoinjector was originally invented in the 1970s to help protect soldiers in the event of chemical warfare (Figure 1). In 1987, the EpiPen (Mylan, part of Viatris, PA, US) for emergency adrenaline (epinephrine) delivery was approved by the US FDA, typically for intramuscular delivery, and did not include a prefilled syringe (PFS). It was followed by other emergency applications, such as for naloxone and glucagon delivery.
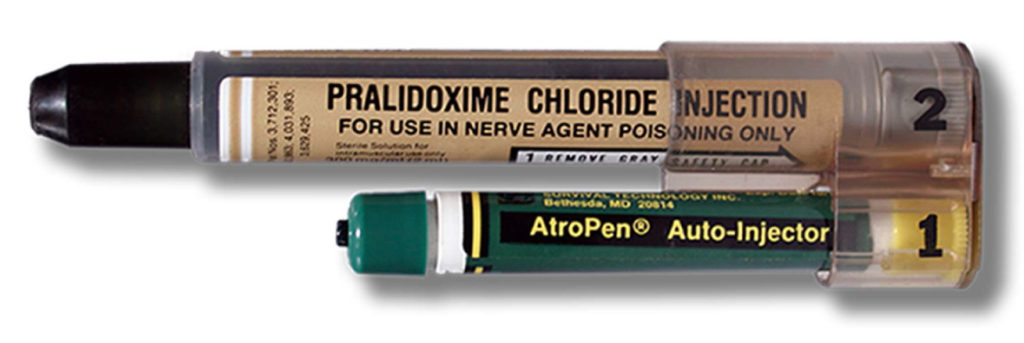
Figure 1: MARK I nerve agent antidote kit.
In the mid-2000s, following a dramatic increase in the number of frequently dosed biologics, autoinjectors for regular home use were developed and introduced. Disposable autoinjectors for Amgen’s Aranesp (darbepoetin alfa; long-acting erythropoietin) and Neulasta (pegfilgrastim) launched around 2005, although neither stayed on the market for very long, with devices for AbbVie’s Humira (adalimumab) and Immunex’s Enbrel (etanercept) launching in 2006. Much of the design work was focused on improving the patient experience, primarily via a reduction in the number of user steps and by improving safety features – such as hidden needles and automatic needle shields.
“Autoinjector growth will be propelled by patent expiration of biologics, leading to the need for more differentiation, potentially including differentiated autoinjector design.”
In the 2010s, human factors testing also became a major driver for design improvement, propelled in part by the FDA and other regulatory bodies placing emphasis on human factors across all medical devices.2 Examples include the incorporation of multiple indicators (visual, audible, tactile) to signal dose initiation and end-of-dose. Patient training materials – such as instructions for use, online videos, talking trainers, realistic device trainers – have all been improved dramatically over the last ~20 years.
Overall, two common modes of injection actuation arose – push-button and push-on-skin. The two-step push-on-skin has become the more popular approach today. Furthermore, some devices have a stationary PFS with the needles inserted into the injection site by the patient, while in others the PFS is shuttled within the device for automatic needle insertion actuated by the patient. Reusable and disposable autoinjectors were both introduced but, over time, disposable has become the most common modality due to ease of use, easy disposability and reliability, as well as lower upfront cost, fewer reimbursement complexities and many therapies not being injected daily or weekly.
Reliability and PFS Compatibility
As the use of autoinjectors grew in the late 2000s and 2010s, so did incidences of glass syringe breakage and malfunctions. Indeed, one of the very first autoinjectors, for Neulasta, was recalled in 2006 because of incomplete injections/slow injection times.
Use of the original glass prefilled syringes (PFSs) was never foreseen in an autoinjector. Hence, ensuring PFS compatibility became a major point of emphasis. Various approaches to modifications in the design of both autoinjectors and PFSs have improved the reliability of autoinjectors,3 and continue to do so.
“Development of devices leveraging alternate power sources such as compressed gas or electromechanical systems can be expected, due to their ability to deliver highly viscous drugs in a small volume (<2.25 mL).”
Viscous and High-Volume Drugs
The increasing viscosity and/or volume of pharmaceutical formulations has been a well-recognised trend over the last several years, driven by a number of factors, such as the move to high-strength biologics, long-acting therapies, the shift from intravenous (IV) to subcutaneous (SC) administration, etc. In addition, many patients do not always wait for their biologic medications to reach room temperature prior to use, which effectively increases formulation viscosity.
Conventional autoinjectors have struggled to inject these drugs due to the high forces required. This has led to the development of new autoinjectors with increased spring strength, but they, in turn, initially experienced quality/reliability issues, such as syringe breakage, device malfunctions and increasing device size. In addition to considering increasing the number of injections, mitigating approaches were taken to strengthen the PFS accordingly and expand available needle sizes (<25G), while also increasing the drug volume to 2.25 mL to help reduce the viscosity (and therefore the force required). However, this has generally resulted in less user-friendly devices – often with larger form factors, higher weights, physical recoil, loud sounds and uneven delivery rates. It is possible to reduce the size of a device by moving away from automatic needle-insertion to manual.
These drugs also tended to have longer injection times (over 15 seconds) to help avoid patient pain and injection site leakage associated with larger delivery volumes. Long wait time was seen as a potentially major negative for hand-held delivery devices and, in part, this has given rise to a new category of devices – wearable injectors – that can help address some of these needs by delivering even higher volumes over a longer time period. However, these too come with their own set of cost, reformulation, disposal and user challenges.
An alternative approach has been to move away from spring-based autoinjectors to alternate power-sources.4 This has included gas-based autoinjectors, which typically can deliver very high forces yet still offer a smooth delivery-rate and a compact size. Despite their many advantages, however, adoption of gas-based autoinjectors has been low to date, perhaps due to reliability and cost concerns and the potential for the release of greenhouse gases in some instances (similar to concerns raised with inhalers).5 Electromechanical devices, such as the AutoTouch device for Enbrel, have also been developed, which offer similar benefits, as well as the potential to integrate connectivity and other digital features, but tend to have a high-cost, large size and need to be reusable, going against the prevailing single-use disposable trend.
Platform Autoinjectors
Development of the early autoinjectors for specific drugs was a highly elongated process. As the biologics revolution continued into the 2010s, and more and more molecules entered the pharma pipeline, speed to market was key – pharma customers no longer wanted devices to be the rate limiting step, especially for drugs such as generics and biosimilars.
Autoinjector manufacturers have responded by introducing platform products, which consist of base devices that can be relatively easily customised to accommodate different specifications within a predefined standard range. This has dramatically cut development times and costs. Pharma companies also tend to view platforms with at least one commercially launched product as “de-risked” options, further increasing their appeal.
CURRENT AND FUTURE MARKET DRIVERS FOR AUTOINJECTORS
Trends within the pharma pipeline will ultimately have a major influence on the next generation of autoinjectors. These include the continued shift towards less frequent dosing and more viscous/high-volume drugs, the emergence of immuno-oncology drugs, increased shift of therapy to home settings, subcutaneous rare disease products and drugs with variable dosing needs. Additionally, autoinjector growth will be propelled by patent expiration of biologics, leading to the need for more differentiation, potentially including differentiated autoinjector design. The continued drive to switch from IV to SC, especially in oncology, and conversion from lyophilised to liquid-stable formulations will expand the autoinjector market. High-strength biologics and longer-acting products will drive the need for autoinjectors capable of injecting more viscous formulations.
Pharma’s strategic needs are also shifting, with a greater emphasis being placed on sustainability6 and cost-effectiveness, as well as disease management, through integrated solutions and interventions, and a desire to gather real-world data and evidence about their drugs.
Higher Volume Devices, Longer Hold Time
Currently, the majority of marketed autoinjector products instruct patients to wait up to around 15 seconds before removing them from the skin. However, there is some evidence to suggest that longer delivery times, and therefore higher volumes (2.25–5 mL), could be acceptable for autoinjectors.7 Indeed, recently 5- and 3-mL cartridge-based autoinjectors were offered for testing. The anti-migraine autoinjector product Ajovy (fremanezumab) from Teva was recently approved in the US with a 30-second hold/dwell time (including 10 seconds after the end of injection).
For even higher volumes (>10 mL), it is likely that wearable devices will still be required, and several devices are in development to cater to this need.
Alternate High-Power Sources
Although the use of springs for higher-volume delivery may be acceptable, patient preference will likely always be for shorter injection times. Therefore, development of devices leveraging alternate power sources, such as compressed gas or electromechanical systems (including micro-electromechanical systems), can be expected due to their ability to deliver highly viscous drugs in a small volume (<2.25 mL). If electromechanical platforms are considered, reusability may be necessary to address cost and disposal challenges.
Reusable Devices for Frequent Dosing and Compliance Monitoring
The growing focus on sustainability and a desire to incorporate electronic/connectivity features may also facilitate a partial shift towards reusable devices in the future. This trend is more likely to take hold with drugs that require frequent dosing (e.g. weekly and daily), where waste from disposable devices is high and a need to ensure regular dosing adherence is key, such as with BETASERON (interferon beta-1b) BETACONNECT (Bayer). For less frequently dosed drugs, for which there is a major trend, the case for reusable devices is not as strong – cost per dose for a reusable device would be relatively high, and the waste difference between one large reusable device with electronics versus a handful of single-use devices is not as stark. It should also be acknowledged that there are strategies to minimise the environmental impact of disposable, including within the supply chain.
Enhanced Ease of Use
While autoinjector devices are generally acknowledged to be easy to use, room still exists for them to be improved further. The need is perhaps greatest for new, injection-naïve users,8 and it is likely that users who inject infrequently will also require more support. Training programmes, enabled by connectivity, online guides and tools, will continue to close the gap, but device enhancements could also play a role.
One example is preventing “wet-injections”, where a user removes the device from the skin too early before the full dose is delivered. The impact of wet-injections is amplified with less-frequent dosing, as a significant period (perhaps months) of treatment may be missed. In some cases, the device is removed early due to patient misunderstanding, and the introduction of digital apps and connected devices that facilitate better time counting (in training and in real-time) can help, as would true end of dose indicators. It is possible that the user may accidentally remove the device prematurely from the skin, or even deliberately if discomfort is experienced, and the chances of this increase with the longer injection times necessitated by viscous drugs, as such, additional innovations may be required.
Connectivity Enabling Disease Management
Connected devices and related software offer the potential to enhance disease management through data collection and providing real-time insight to patients, as well as enabling connection to support services from providers and gathering real-world data for subsequent analysis. Most progress in this regard has been made in diabetes, with integrated insulin pens already on the market to alleviate the complexity of disease management. Medication is another focus area. Companies that offer an integrated solution for diabetes are setting a template for other disease states to follow in due course, with connected injection devices playing an important role. As a result, many device manufacturers are developing connected versions of their autoinjectors, and it is expected that these will be important next-generation products.
| Needs | Current Solution Examples | Future Innovation Examples |
| Reliability/PFS Compatibility | • Improved device/PFS designs • Improved siliconisation • Polymeric PFSs |
• Enhanced designs for next-generation devices/containers |
| Human Factors/Usability | • Multiple user feedback/indicators • Simple two-step devices • Enhanced customer training |
• True end-of-dose indicator • Connected training devices • Wet injection prevention |
| High Dose Volume | • 2.25 mL hand-held autoinjectors • 3.5 mL wearables |
• Handheld >2.25 mL (e.g. with longer hold time) • Wearables >3.5–25 mL |
| High-Viscosity Formulations | • Wider bore/thin-wall needles • Enhanced springs |
• Electromechanical • Alternate power sources • Longer hold time • Polymeric PFSs |
| Faster, Lower-Risk Device Development | • Platform devices – fixed dose, with limited customisation | • Platform devices – variable or fixed dosing, with wider range of customisation |
| Disease Management | • Compliance monitoring | • Multiple sensors/monitoring • Integrated interventions |
| Sustainability | • Disposal management systems | • New materials/reusable devices • Supply chain efficiency |
Table 1: Summary of autoinjector achievements and potential future innovations.
All-Encompassing Platforms
Today, relatively few autoinjectors are developed that are not ultimately part of a platform. However, the predefined specification ranges are often relatively narrow, meaning autoinjector companies may still offer a wide array of device platforms to cover all potentialities, and a pharma company may well need several platforms to cover their portfolio needs.
The industry is slowly moving towards more comprehensive platforms. Moving forward, this trend is expected to continue, with single platforms able to accommodate a wider range of drugs by offering:
- Delivery of a broad range of viscosities (up to 3000 cP). This helps with standardisation of manufacturing infrastructure, while providing flexibility (drug viscosities and/or needle gauge can change during development) and accommodating user-driven issues (“non-viscous” drugs can present as “viscous” if the drug is injected too soon after removal from refrigeration).
- Wide range of customisable injection speeds.
- Variable- and fixed-dose options.
- Cartridge-based options.
- Full-range of dosing volumes right down to the microlitre level. This is useful for paediatric applications that often require smaller volumes, as well as for dose ranging during clinical trials.
In conclusion, the ever-evolving biologics landscape is creating the need for more innovation, and the future will likely see the needle-based autoinjector category continue to evolve significantly (Table 1).
Acknowledgements to Jayshree Srinivasan for her research and to Mathias Romacker for his insights that supported the creation of this article.
REFERENCES
- Sonar A, Sumant O, “Allied Market Research, Autoinjectors Market by Type (Disposable Autoinjectors and Reusable Autoinjectors), Application (Rheumatoid Arthritis, Anaphylaxis, Multiple Sclerosis, and Others), and End user (Homecare Settings and Hospitals & Clinics): Global Opportunity Analysis and Industry Forecast, 2020–2027”. Allied Market Research, Sep 2020.
- “Guidance For Industry: Applying Human Factors and Usability Engineering to Medical Devices”. US FDA, Feb 2016
- Bankston T, “The Value of a BD Integrated System for Combination Products”. ONdrugDelivery Magazine, Issue 79 (Oct 2017), p52–57.
- Welch W, “Advancements and Evolution of Power Sources in Drug Delivery”. ONdrugDelivery Magazine, Issue 133 (May 2022), pp 57–60.
- “Our position on: Respiratory Products and Global Warming”. PDF GSK, Oct 2021.
- Earl M, “So far so good: Maintaining the momentum of progress for sustainable policies”. ONdrugDelivery Magazine, Issue 129 (Feb 2022), pp 40–42.
- Schneider A, “How Long Can You Hold the Device Against the Skin? Insights From an Empirical Study Using Hand-Held Autoinjectors” ONdrugDelivery Magazine, Issue 113 (Oct 2020, pp 8–11.
- Lageat C et al, “Formative and Validation Human Factors studies of a new disposable autoinjector for subcutaneous delivery of chronic disease therapies.” Expert Opin Drug Deliv, 2021, Vol 18(11), pp 1761–1775.

