To Issue 131
Citation: French T, “Breaching the Blood-Brain-Barrier with Particle-Based Nasal Delivery Systems”. ONdrugDelivery, Issue 131 (Apr 2022), pp 27–30.
Tom French talks about the advantages of the nasal drug delivery route and crossing the blood-brain-barrier to treat central nervous system diseases.
“Recent developments in particle engineering have resulted in the ability to produce a dosage form that overcomes all the previously mentioned obstacles. It is now possible to produce powders of significantly reduced particle size.”
New developments in particle science and powder filling technology have enabled pharmaceutical researchers to explore a new range of nasally delivered drugs. Administration via the nasal route means that these drugs can successfully cross the blood-brain-barrier (BBB) to address the as-yet unmet medical needs of patients suffering from a variety of central nervous system (CNS)-based diseases.
Within the pharmaceutical and medical sector, there are a number of well-known and long-established drug delivery routes. These include intravenous, subcutaneous, pulmonary and the use of oral solid dosage forms. In addition, nasal administration is currently witnessing significant growth. Delivering drugs via the nose has shown remarkable advantages, including a rapid and high systemic availability, the avoidance of first pass metabolism by the liver and the possibility of targeting drugs directly from the nasal cavity to the brain.
Furthermore, by avoiding the gastrointestinal (GI) tract, patients generally experience fewer side-effects. However, getting the dose right is critical. Compared with the lungs and the intestines, for example, the nasal cavity has limited ability to accommodate dose absorption through the mucosal membrane. As such, any formulation must be relatively small in size and highly concentrated to be successful.
Despite these technical challenges, nasal administration presents opportunities to address large patient populations that traditionally have had very few treatment options. Key therapy areas with unmet needs include Parkinson’s, multiple sclerosis, Alzheimer’s, dementia and a number of other CNS-based diseases. Most treatments are only able to address the symptoms and provide palliative care because they are unable to cross the BBB.
However, recent developments in particle engineering have resulted in the ability to produce a dosage form that overcomes all the previously mentioned obstacles. It is now possible to produce powders of significantly reduced particle size. In addition, these can be formed with hollow centres, such that the overall weight and density are also reduced – often by an order of magnitude. Compatible with traditional spray drying technologies, these particles can be produced using spray-freeze drying and even 3D printing. Of key importance, however, is their ability to cross the BBB, avoid the GI tract (reducing side-effects) and remain in the limited area of the nasal cavity long enough to be absorbed.
“For the device to deliver the concentrated microdose both accurately and repeatedly to the small surface area of the nasal cavity, it’s imperative to protect the very delicate particles and ensure that their physical characteristics are unaffected.”
The significant differences between human and animal physiologies often makes it hard to correlate data between them, particularly in terms of stability testing, but the new powder particles have successfully demonstrated their ability to be stored safely in nasal delivery devices. 3P innovation is currently working on several novel vaccine delivery systems, including a nasal one. This means that, in powder form, the company can eliminate all the previously necessary cold chain requirements, making distribution easier and more cost-effective. There are challenges to overcome; the drug needs to be present in the right area of the nose long enough to be efficacious; compared with the huge surface area of the GI tract or the large volume of the lungs that’s normally available for respiratory drugs, the surface area of the nose is very small. As such, the dose needs to be small, concentrated and accurate. Beyond the benefits already mentioned, the novel particles also exhibit higher levels of stability compared with, for instance, classic large molecule drugs, parenterals and biologics.
Being developed as a single-use self-applied vaccine, it can be sent out all over the world and enable people to inoculate themselves. In light of the recent and ongoing covid-19 pandemic, this is a significant breakthrough that could positively affect thousands if not millions of lives. The delivery system (a plastic nasal inhaler) is also more sustainable than complex injection systems (vials, stoppers, crimps, syringes, needles, etc).
Yet, because these particles are both small and hollow, they are also very delicate. Of paramount importance for their effective use is the ability to process them without affecting their characteristics. Because the dose size, concentration and weight are all critical, it is necessary to weigh every single dose and minimise any work induced by the processing equipment. Unfortunately, the physical properties of the powders that make them efficacious also make them unsuitable for processing by conventional powder-dispensing technologies. Most dosing technologies influence powder properties due to the fact that they rely on compaction or insertion – involving the application of energy or force – which could potentially damage a friable or fragile formulation.
Furthermore, as well as being susceptible to water, these particles have a high surface-energy-to-mass ratio, meaning that they are highly cohesive, flow poorly and are prone to compaction. Their hydroscopic nature causes surface adhesion, which, along with a very low bulk density, adversely impacts device performance. As mentioned prior, typical dosing technologies can compact the powder and damage the particle structure. So, given that avoiding compaction would result in less complex device requirements, a low-shear, non-compacting dosing technique was needed, otherwise nasal delivery of such powders could be rendered infeasible without a suitable powder-dispensing technology.
What is required is a powder dispensing technology that is gentle (low shear and compaction) and ideally gravimetric in nature, such that every dose is weighed precisely. These powders cannot be compacted or crushed. For the device to deliver the concentrated microdose both accurately and repeatedly to the small surface area of the nasal cavity, it is imperative to protect the very delicate particles and ensure that their physical characteristics are unaffected. (Figure 1).

Figure 1: Close-up of 3P’s Fill2Weight gravimetric filler in their R500 Robotic Capsule Filler.
“Unlike conventional systems, Fill2Weight adjusts for changes in a powder’s physical properties, including drying, taking on moisture (caking), aerating or densifying.”
There are not many technologies that can dose these powders into devices at such small dose sizes without impacting the particles and even fewer that can dose them accurately and repeatably enough to produce a manufacturing process that is economically viable. 3P innovation’s patented Fill2Weight technology, which was originally developed for similar inhaled particles, enables nasal delivery of these engineered particles. It is no accident that the company has seen significant interest in its equipment for nasal drug delivery applications.

Figure 2: Fill2Weight – 3P’s award-winning gravimetric platform dosing technology.
WHAT IS FILL2WEIGHT?
3P innovation’s proprietary gravimetric platform dosing technology employs a multiparameter control system to ensure consistent and controlled flow, which is essential to achieve accurate dosing (Figure 2). By design, very little powder is contained within the dispensing head, such that the energy required to control the flow of the powder is minimised. Conventional volumetric systems (dosator/vacuum, dosator/tamping, pin/auger) compress the powder to achieve accurate dosing. Unfortunately, this leads to a level of consolidation. This is less than ideal for an inhaled or nasally delivered powder and, worse than that, fragile particles can be badly damaged.
At a basic level, Fill2Weight is a pin in a hole – a simple valve. The pin is lifted to open the valve. Unfortunately, most powders would simply bridge above the opening in the valve. However, the pin spins and has miniature stirrers just above the valve that prevent bridging and, even though most powders would still bridge here, the tip of the hopper is connected to a piezoceramic actuator that generates controlled vibration to prevent bridging. This is very similar to tapping a salt cellar to promote flow.
The pin opening, stirrer speed and vibration (frequency and amplitude) are all accurately controlled in response to feedback from a high-speed precision weighing device. Fill2Weight is controlled such that the system can initially open the valve to a high value to dispense inaccurately and quickly, then, as the target weight is approached, the valve can close to a slower and more predictable flow. This “two-slope” control strategy enables both speed and accuracy. The very localised application of vibration ensures that powder blends are not segregated or damaged. Unlike conventional powder dispensing technologies, Fill2Weight does not damage spray-dried particles and microspheres, which are ideal for efficacious nasal drug delivery.
Real-time measurements during the dosing process ensure accuracy and, of course, every dose is check-weighed. Increasingly, clinical batches require 100% weight verification, which comes as standard with Fill2Weight. A custom-designed algorithm controls in-process dose parameter adjustments to improve dose consistency throughout the batch to overcome any inconsistencies, such as powder density variations in the feed hopper. Unlike conventional systems, Fill2Weight adjusts for changes in a powder’s physical properties, including drying, taking on moisture (caking), aerating or densifying. Fill2Weight takes these changes in its stride by adjusting process parameters automatically.
Fill2Weight is also scalable – the number of heads can be increased to match production outputs with a need for tech transfer or traditional scale-up. This is why, at the design stage, Fill2Weight was designed to be as narrow as was practical; multiple Fill2Weight heads can be aligned next to each other above a production line in a compact arrangement. Being able to ramp up production from the bench to the clinic is critical for a new drug. Not only that, it can significantly reduce the regulatory approval timescale and expedite the drug-device matching process. These nasal products rely on a suitable drug delivery system that often has to be designed from scratch – they’re frequently brand new and have never been used before (Figure 3).
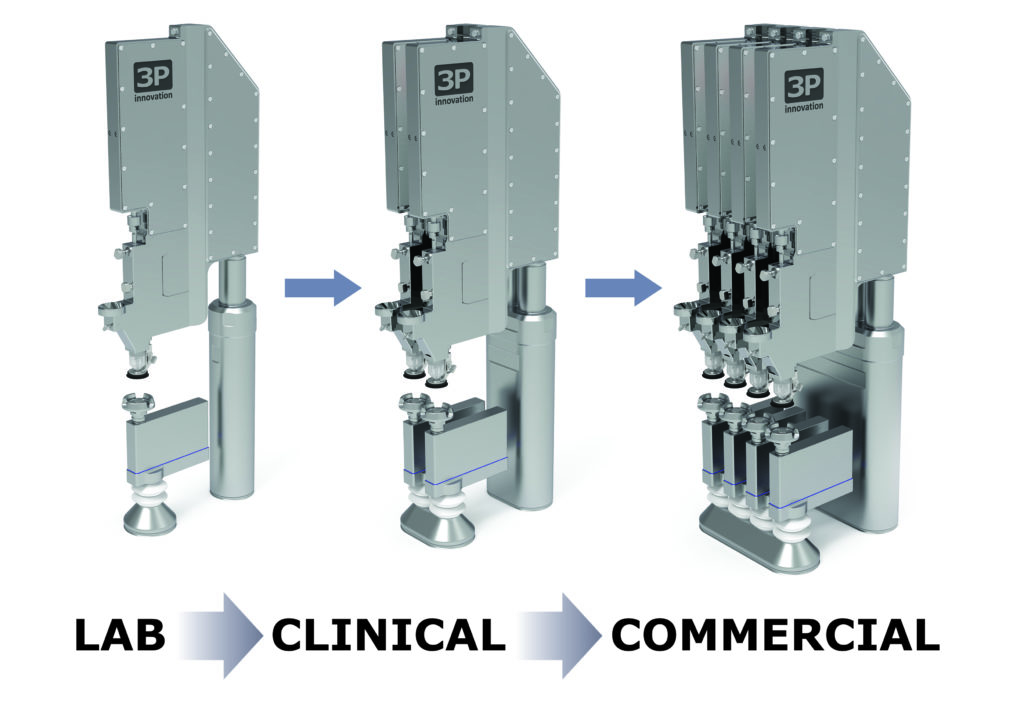
Figure 3: Fill2Weight is flexible and scalable.
“With the use of robots to replace human operators
to expand production capacities rapidly, a small-scale
investment can easily be subsequently augmented to cope
with increasing demand during the clinical trial process.”
DE-RISKING NOVEL NASAL DRUG DELIVERY PROJECTS
The commercial viability of nasal drug delivery has been enabled by novel manufacturing techniques (such as 3D printing), breakthroughs in material science and the development of engineered particles. The last piece of the puzzle is the ability to manufacture cost-effectively at clinical and commercial scale. The right equipment is absolutely necessary to assemble devices and fill this completely new drug delivery system. Companies may be looking for something that doesn’t actually exist yet and need to produce it at small-, mid- and large-scale, which is something that 3P innovation can assist with.
Custom device assembly plays a large part in drug delivery, but successfully transferring a novel product through clinical trials and on to commercial manufacture and assembly at the various scales required can be challenging when there is no prior example to learn from.
De-risking production processes from a very early stage is essential. A balance needs to be reached between planning to scale without committing huge amounts of capital and quickly moving through the various approval phases without purchasing dedicated equipment. Projects are generally broken down into stages to protect the device owner from large capital outlay when significant unknowns exist due to the clinical landscape. However, very few companies can afford to wait 12 months between Phase I approval and implementing Phase II for a machine to be built!
The appropriate use of automation provides the answer. 3P innovation has developed a methodology of scaling automation from manually actuated benchtop equipment for early phase and clinical supply. By thinking about scaling at the outset, these processes can be later scaled-up or scaled-out as necessary for commercial supply. With the use of robots to replace human operators to expand production capacities rapidly, a small scale investment can easily be subsequently augmented to cope with increasing demand during the clinical trial process. Additional tracks can be added over time to multiply outputs and extra machines can be installed to enhance output even further while spreading any perceived risk across multiple manufacturing lines.
CONCLUSION
This article has discussed how unmet clinical needs can be met by nasal delivery. Nasal delivery devices not only provide a convenient method of self-administration but their simplicity leads to improved compliance and adherence. This is particularly important as ever more clinical trials become decentralised. The initial driver of this growth in nasal delivery was developments in particle engineering that enabled superior efficacy. More recently, innovative powder dispensing technology has overcome the challenges associated with manufacture.
These technical advances have paved the way for the use of cold-chain-free vaccines and the treatment of a variety of CNS diseases. These simple devices also require fewer physical resources in their manufacture, leading to more sustainable vaccine delivery. In turn, they also reduce the burden on hospitals and healthcare systems around the world, as a highly trained healthcare professional is no longer required to deliver an injection. Furthermore, not needing patients in a specific location to run clinical trials and having tighter control of dose delivery offers many benefits, including reduced risk of over or under-dosing, less waste and improved compliance. Advantages of the nasal route include avoiding needle phobia and improved adherence with single-use devices, which helps overcome some of the problems associated with geriatric participants.
Yet, to manufacture any kind of drug device combination at scale requires a great deal of time and investment to navigate development, production and commercialisation steps. 3P innovation offers a powerful combination of standard technologies and machine platforms with custom automation skills and methodologies that provide the optimum mix of tailored solution with proven technology for the its customers. 3P innovation is very excited about the future of nasal drug delivery and its benefits for the planet.

