To Issue 131
Citation: Chandra A, Audibert R, “Addressing Systemic Delivery with a Unit-Dose Nasal Spray”. ONdrugDelivery, Issue 131 (Apr 2022), pp 33–36.
Audrey Chandra and Raphaële Audibert discuss the benefits of a unit-dose nasal spray for rapid treatment in an emergency.
Systemic-acting drugs are commonly administered through the injectable route. Rapid onset tends to make injection the most efficient route, especially in crises and life-threatening situations. It allows the medication to enter the bloodstream directly with accurate dosing and higher bioavailability than oral administration as it bypasses the hepatic metabolism.
Injections are invasive so normally require healthcare professionals’ intervention. For instance, intravenous self-administration could be trickier to handle for novice users in a home setting. Moreover, needles may complicate drug administration, especially for patients with needle phobia.
Other delivery alternatives for systemic treatments, and especially nasal administration, are further explored to offer less-invasive treatment, providing the same efficacy. The complex nose anatomy offers various advantages. It avoids the first-pass metabolism, which therefore improves bioavailability. In addition, nasal devices are needle free, enabling easier self-administration, which increases the patient acceptance level. This leads to positive therapy outcomes as adherence and compliance of patients can be improved.
Conventionally, the nasal route (Figure 1) is used to treat chronic conditions, such as allergic rhinitis or sinusitis, by targeting the ostiomeatal complex (OMC) region using multidose nasal spray pumps. Nowadays, the nasal route is also used for the administration of medications systemically by targeting the nasal turbinates that occupy a highly vascularised large surface area of the nasal thin mucosa.
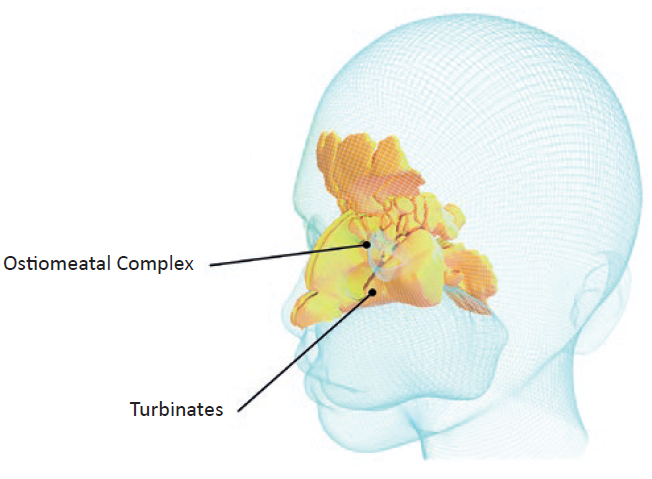
Figure 1: A 3D rendering of nasal cavity regions for local and systemic nasal-spray administration.
“An increasing number of prescribed systemic-acting drugs for acute conditions originally administered in injectable forms have been made available as unit-dose nasal sprays.”
Multidose nasal spray pumps, however, are not well adapted for acute applications. If the device use is sporadic, repriming might be needed before use. Also, a large volume of drug content could be wasted if the device is used only once. Consequently, novel therapies are starting to emerge with precise, ready-to-use unit-dose nasal spray delivery to treat emergencies.
Thanks to the accessibility and simplicity of using nasal sprays, in recent years there has been a clear growing interest in exploiting the nasal route for systemic and acute indications, with many approved market references of unit-dose nasal sprays for various therapeutic areas, according to data from PharmaCircle. For example, a patient with a chronic condition suffering from breakthrough pain is normally prescribed potent drugs, such as opioids, which can lead to addiction and, potentially, overuse. In the case of an overdose, every second counts – an antidote needs to be administered straight away to save the patient’s life.
However, often, actions cannot be executed immediately as healthcare professionals are not directly available to inject patients with the medications intravenously. This could lead to a worsening condition or even death. To prevent this, an increasing number of prescribed systemic-acting drugs for acute conditions originally administered in injectable forms have been made available as unit-dose nasal sprays. Patients and/or their caregivers can then administer a one-shot spray easily and rapidly, if necessary.
Unit-dose nasal sprays can also be used by patients suffering from migraines or seizures to obtain rapid relief without the intervention of a healthcare professional. To ensure patients’ safety, the regulatory bodies impose stringent regulations on alternatives such as nasal spray delivery to ensure it is at least as effective as injection, especially for life-saving drugs. The reliability of the device acts as one of the key elements to save patients’ lives.
ENSURING DEVICE ROBUSTNESS FOR PATIENT SAFETY
Involving patients and end users as early as possible during the device-design process is essential to ensure the device being developed is appropriate. A series of formative studies should take place to optimise device design to meet users’ needs. Device manufacturers must ensure that the design of the unit-dose nasal spray device is intuitive for correct and easy drug administration, making it accessible for patients or caregivers to use without any training. The device must be able to administer the required dose of medication targeting the proper zone of the nasal cavity to enter the bloodstream. Also, the reliability and robustness of the device act as key elements in successful administration, where regulatory bodies require the device to work almost 100% of the time.
TAILORING THE DEVICE FOR A SPECIFIC COMBINATION PRODUCT
It is incumbent on pharma companies to ensure their selected device, in combination with their drug, is appropriate, safe and effective for the targeted population. Nemera works with customers to provide technical support, laboratory testing, human factors and patient experience activities necessary for a successful drug-device combination product development process (Figure 2). This also extends to, wherever possible, optimising the patient experience to create competitive differentiation and to ensure adherence and engagement with patients and clinical stakeholders.
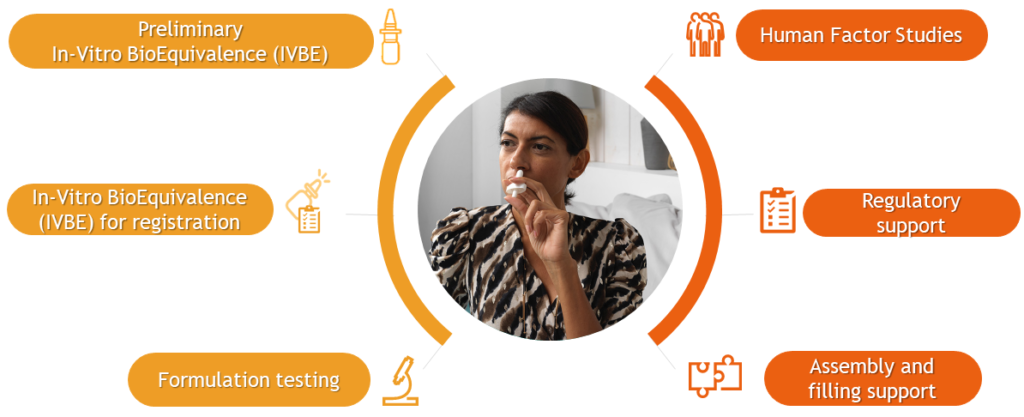
Figure 2: Answering combination product needs with Nemera’s holistic services.
Customers in generic markets might be interested in differentiation wherever possible, as many competitors are targeting the same reference drug or devices. In the case of generics, the regulatory authorities ask systematically if the device used corresponds to the criteria of human factors studies evaluations by comparing its user interface – inclusive of all materials that a user would interact with – with the originator.
“The spray characteristics obtained must provide correct shot weight, droplet size distribution, spray pattern and plume geometry, among other things.”
One of the regulatory requirements is to perform user threshold analysis to understand the risks and therefore mitigate them. User-interface evaluations include labelling comparisons, comparative task analysis and physical comparison of the delivery device constituent parts. Usually, device manufacturers should conduct preliminary user threshold analysis to mitigate the risks found in the steps of use and in the physical comparison analysis, ensuring equivalent use of the device from the user perspective without compromising its safety; while the labelling comparison analysis should be completed by the pharma company. The results of preliminary user threshold analysis documentation by the device manufacturer can be used and leveraged by pharma laboratories to complete labelling analysis, thus filing full user threshold analysis to the US FDA. Going the extra mile, Nemera’s front-end capabilities will be able to support specific combination product formative studies to be filed in specific dossiers to the regulatory authorities.
STATE-OF-THE-ART LABORATORY CAPABILITIES FOR COMBINATION PRODUCT TESTING
For successful administration, the drug formulation must have a high rate of mucosal and blood absorption while the device must deliver the formulation with consistent performance in addition to an optimised user interface. Combination product performances can be analysed by testing the nasal pumps together with the targeted formulations. The spray characteristics obtained must provide correct shot weight, droplet size distribution, spray pattern and plume geometry, among other things. The formulation’s properties influence spray characteristics that need to be tested to obtain consistent performance. Thanks to Nemera’s state-of-the-art laboratory capabilities, it offers formulation testing services to help pharma companies’ formulation development.
In the case of generics, the drug-device combination product needs to prove its bioequivalence in comparison with the originator. As first trials, Nemera offers preliminary in vitro bioequivalence (IVBE) studies to check the status of the generic drug and device combination compared with the originator. These tests can be redone during formulation development. Once the formulation is finalised, Nemera can also perform full IVBE studies for registration, which can then be included in the final dossier submitted to the authorities to prove IVBE of a specific nasal spray formulation with Nemera’s device. The company’s capability in method development assures reproducible device performance testing and its expertise allows it to perform statistical analysis for registration in different geographical areas.

Figure 3: UniSpray offers safe and
intuitive handling for efficient drug delivery.
FACING EMERGENCIES WITH A ROBUST UNIT-DOSE NASAL SPRAY
Nemera’s UniSpray (Figure 3) delivers a single-metered 100 μL dose spray and can be used for new, repurposed or generic drugs. UniSpray has gone through different human factors studies, design verification and processes to ensure reliability and robustness of the device, assuring patients’ safety and ease of use, and complying with the regulatory exigence with no risk of accidental activation.
This one-shot nasal spray is a ready-to-use primeless device with 360° functionality, enabling one-handed activation in an emergency. To ensure the correct use of the device, it offers an ergonomic design and intuitive usage.
To accelerate time to market for pharma companies, UniSpray is compatible with existing marketed primary drug containers and is adapted to fit conventional filling lines as well. In line with this objective, for generic drugs, the preliminary bioequivalence of selected molecules is performed by Nemera, which generates preliminary documentation to ensure device robustness, assuring spray characteristics equivalence and consistent performances. Customers then complete the process, based on their formulation. For new or repurposed drugs, spray performance adjustment can be done to ensure drug administration efficacy.
CONCLUSION
To bring drug-device combination products to market successfully, Nemera’s expertise in human factors studies can help and support customers from the user’s perspective to meet regulatory requirements. This is done by assessing the combination product with specific targeted populations according to the applications and therapeutic areas.
The right combination of device robustness and ease of use, as well as drug efficacy, plays a crucial role in nasal spray administration to deal with emergencies. Nemera’s long-standing experience in developing and manufacturing complex multidose nasal devices has successfully brought more than 70 combination product references to the market across the globe, ultimately to serve patients. The company leverages its legacy and proven track record in multidose pump development and manufacturing for UniSpray’s development targeting systemic and acute delivery intranasally.
Through capabilities in design research, human factors engineering, user experience design, engineering, lab services and regulatory support, Nemera is uniquely positioned to offer all the support that customers require through an integrated device platform and service programme.
Thanks to increased early-stage development capabilities, now Nemera teams can be a single partner for device platforms and integrated services – from front-end innovation, design research, human factors and design engineering to strong late-stage development, as well as clinical and commercial manufacturing capabilities.

