To Issue 136
Citation: Tunkel M, Sapatnekar N, “Holistic Development and Industrialisation of Drug Delivery Devices and Combination Products”. ONdrugDelivery, Issue 136 (Aug 2022), pp 39–42.
Mark Tunkel and Niyati Sapatnekar discuss Nemera’s holistic approach to development and industrialisation.
“The complexities involved in selecting or developing the correct device for a combination product have never been more challenging.”
Doctors have always known that healthcare isn’t a one-size-fits-all business – it requires a personalised, value-based approach.1 Whilst the healthcare landscape continues to evolve, accelerated by the covid-19 pandemic, it is increasingly important to keep up with the surrounding changes, including climate change, geopolitics and vulnerable populations.
Over the past few years, new insights in biology, chemistry and engineering have led to the discovery of new molecules and drugs. This, in turn, has increased the necessity of well-adapted drug delivery device solutions to ensure their maximum efficacy, as well as the best patient outcomes. Staying on top of healthcare trends is critical not only for well-established drug providers but also for those smaller players intervening in and around the space. Industrialising new devices and introducing them to the market requires a rigorous process that navigates a variety of factors and strengths in contract development, and manufacturing expertise that can be applied in a flexible manner.
AN INCREASINGLY COMPLEX LANDSCAPE FOR COMBINATION PRODUCTS
The complexities involved in selecting or developing the correct device for a combination product have never been more challenging. A convergence of trends, including significant growth in the global drug development pipeline, the need for more complex delivery devices to address targeted applications and drug attributes, and increased migration of care from the clinic to self-administration in home settings have driven demand for a wide range of solutions. This is coupled with a crowded and competitive landscape in the biologics, biosimilars and generics segments, where speed to market, differentiation, patient centricity and value creation are critical.
“It is critical to fully understand the patient journey as well any
related clinical processes.”
Beyond the patient, this includes an ecosystem of stakeholders including not only providers (healthcare professionals) but also health systems, payers (to consider factors such as value-based care) and regulators, as the market for product introduction and the intended filing approach can affect device selection and development strategies. These factors then need to be considered within the available or emerging technology landscape.
Therefore, developers need to adopt a holistic approach to developing a device strategy that is focused on the entire combination product, spans development stages and requires specialised expertise at every step of the process – all balancing a variety of factors and influences that need to be considered. This leads to better near- and long-term decision making across the lifecycle of drug products (Figure 1).
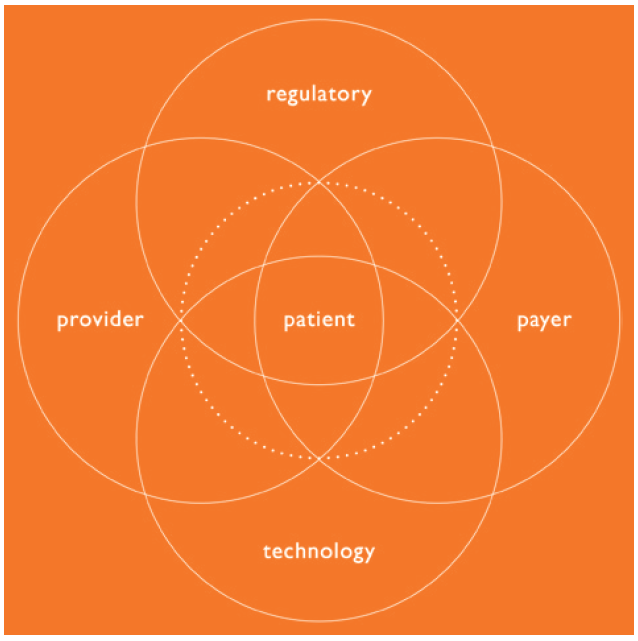
Figure 1: The combination product ecosystem.
INTEGRATING THE VOICE OF THE PATIENT INTO DEVELOPMENT
At the earliest stages of establishing the functional requirements and user needs for a new device application, it is critical to fully understand the patient journey, as well any related clinical processes. Use of a method called “applied ethnography” can achieve this goal. This relies on interviews and in-context observations of practices, processes and experiences within the patient’s home or use environment.
Potential use cases are looked at broadly, beyond the administration event or complying with instructions for use. This starts from when a patient is diagnosed and continues through the entire process of receiving their device, then preparing and administering their medication, to final disposal of the device, as well as considering the times in between treatment, to understand how the process changes over time and fully how frequency of administration may affect the patient experience.
It is equally important to gain an understanding of the experience of healthcare professionals to consider relevant settings in clinical environments. This is important in applications where care is being provided in both at-home and clinical environments; consider the migration of care from settings with significant support systems, such as an oncology ward, to an environment of self-administration where clinical personnel are not present and the burden of support falls to a family member or caregiver.
The outputs of this process include patient journey maps, clinical process maps, an understanding of prioritised user needs and values and pain points that can be leveraged to improving the patient and provider experience.
These outputs support meeting needs holistically while also assessing the technology landscape to identify existing intellectual property (IP) or platforms that may be fit for the intended device function and combination product attributes. Alternately, this can lead to the need for the development of a novel delivery system, for which this foundation can be used to establish user needs and functional device requirements (Figure 2).
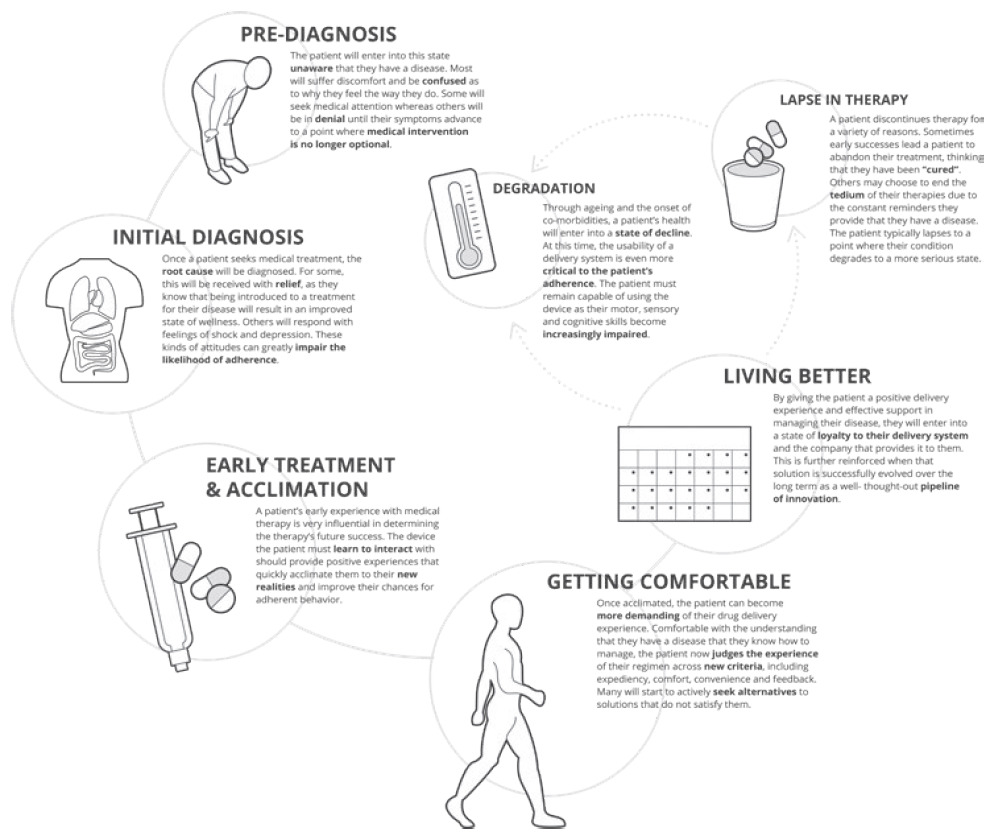
Figure 2: Key milestones along the patient journey and implications for delivery device design.
INNOVATIVE DEVICE DEVELOPMENT & PRECLINICAL SUPPLY
In applications that are not best suited for existing platform devices, there is an opportunity to develop a new device with a customised user experience that addresses the whole of the device usage process and broader patient journey, and also results in an original IP that is owned by a developer. This requires an innovative design development partner with broad experience and technical functions with various devices and a wide range of patient populations and applications. Multiple scenarios for optimising care through user interaction or technical function can be considered, as well as design for sustainability. In some cases, background “technology bricks” can be used as a base for development to accelerate development cycles and to simplify the development of critical technical functions and navigation of the IP landscape.
Ideally, there should be an option for fast-to-completion, small-series production for internal development or registration with agile assets for manufacturing and assembly that integrate the knowhow of a proven manufacturing partner and planning for later stages.
This results in innovative devices that have a manufacturing strategy integrated from the earliest stages of development for all necessary supply. This ensures innovation is balanced with user based and technical risk identification and mitigated early to reduce the risk of transition to manufacturing and end users upon market introduction.
SAFE, EFFECETIVE AND DIFFERENTIATED COMBINATION PRODUCTS
Any device development programme must include human factors and patient experience activities. It is critical to ensure that the selected device, in combination with the drug, is appropriate, safe and effective for the target population and suitable for the chosen regulatory strategy. This also extends to optimising the patient experience to create competitive differentiation and ensure adherence and engagement with patients and clinical stakeholders.
A good example of this approach might be the consideration of a biosimilar application where competitors are targeting the same reference drug and devices. Alternately, for new drug applications and new device development programmes, the company needs to project what a future use case might look like and anticipate areas of risk to ensure that development is tailored to mitigate them. In both instances, the company needs to be sure that it is addressing the defined user group populations and early use-related risk analysis activities to define the human factors and usability programme.
As part of the overall regulatory management strategy for the device in the relevant markets, human factors must be addressed from planning and initial risk analysis to conducting formative and summative usability testing for the device. This includes the production of human factors engineering report documentation for use in regulatory submissions as part of the larger documentation set required. This also includes instructions for use and value-added packaging, as well as leveraging digital-health-related add-ons to support patient engagement and adherence. It is crucial this is all completed holistically.

Figure 3: Agile industrialisation.
CUSTOMISED INDUSTRIALISATION AND COMMERCIAL MANUFACTURING
Every application is unique, so process development and requirements for clinical and large-scale manufacturing need to be aligned to these specific goals leveraging broad experience with many device types. This includes definition and selection of the proper assembly and automation approach, as well as a global approach to mould development and sourcing with a proven supply base. Custom approaches for all aspects of the manufacturing process that can deployed at a variety of global manufacturing sites should be considered.
A diverse team of experts should ensure that all learnings from the development to date are integrated in the most effective way possible. Any initial approaches for preclinical or small-series manufacturing can later be scaled up to a high-volume manufacturing setup or ideal manufacturing location with the appropriate assets and expertise once initial development objectives have been met. This approach speeds up time to market and enables deferment of capital expenses. The result is a reliable and repeatable process that is as unique as the device that has been developed (Figure 3).
BENEFITS OF PARTNERING WITH AN INTEGRATED SERVICE PROVIDER
Nemera’s integrated development, consulting and manufacturing services allow customers to achieve the outcome of a successful regulatory submission and commercial launch of a safe, effective and differentiated combination product with a single partner applying an agile process across the device and combination product value chains. This support is provided from definition of the programme and requirements through to sustained delivery of commercial supply. This will drive patient centricity, reduction of risk and decreased time to market.
This approach can be applied to Nemera’s established IP platforms or to organic development and allows customers to focus on their core business related to their drug assets. Furthermore, Nemera’s procurement teams strive to embed sustainable criteria in their approach with suppliers and other stakeholders from the earliest stages of the process. This helps limit the impact on the planet.
REFERENCE
- “2022 Healthcare Industry Trends That Will Make a Difference”. UCF Online, accessed Aug 2022.

