To Issue 137
Citation: “Mikron Automation: a Leader in the Complex Assembly of On-Body Injectors”. ONdrugDelivery, Issue 137 (Sep 2022), pp 33–35.
Mikron Automation sets out its expertise in the assembly of on-body injectors, a field requiring exceptional precision and innovative approaches, as well as discussing how Mikron works with its partners from the very earliest stages of device development to help ensure projects are completed efficiently and successfully.
“Similar to the way autoinjectors underwent lengthy development periods before experiencing their recent boom in demand, on-body injectors are still an emerging technology at present.”
Mikron Automation is a leading partner for scalable and customised assembly systems and has an excellent track record in the pharmaceutical industry, particularly in the complex assembly of drug delivery devices. With more than 3,900 machines designed and installed worldwide (Figure 1), the Swiss assembly machine designer and manufacturer continues to demonstrate its commitment to innovation with its implementation of assembly processes for on-body injectors, which are used in the treatment of diabetes and other chronic diseases. These small injection devices adhere directly to the skin and therefore require Mikron to address several assembly challenges – critically, the handling and manipulation of the highly miniaturised parts that make up the device and the ability to perform simultaneous engineering with customers when the product is still undergoing design changes.

Figure 1: A fully automated Mikron assembly line.
“Particularly innovative and the subject of numerous patents, on-body injectors are very complex in their assembly and require several parts, some of which are no larger than a few tenths of a millimetre.”
PUSHING THE LIMITS
Mikron Automation is well-versed in the diabetes market, having produced numerous assembly systems for pen injectors and other connected devices. However, similar to the way autoinjectors underwent lengthy development periods before experiencing their recent boom in demand, on-body injectors are still an emerging technology at present.
“We began receiving the first customer requests for this type of device in 2013–2014,” recalls Flavio Pointet, Key Accounts Manager at Mikron Corporation Denver (CO, US). Mikron is well-known in the pharmaceutical market for the precision of its machines, the quality of its systems and its expertise in complex assemblies. This reputation led to the company being approached for the first on-body delivery device projects. Although Mikron’s expertise was already well established, embarking on the assembly of a new type of highly innovative device is always a challenge. “It required us to go even further in our capabilities to implement miniature part assembly processes to be able to achieve these previously unattainable high-precision systems”, acknowledges Jean-François Bauer, Head of Marketing and Business Development at Mikron Automation.
EXTREME MINIATURISATION
Medical device manufacturers design on-body drug delivery devices to be as small as possible. This technology is pushing the boundaries of miniaturisation so that the patient can benefit from the device without it obtrusively impacting their daily life.
Particularly innovative and the subject of numerous patents, on-body injectors are very complex in their assembly and require several parts, some of which are no larger than a few tenths of a millimetre. For example, some silicone seals have an external diameter of less than 1 mm. In addition to their soft and sticky material, their small size makes all gripping, placement and control operations during the assembly process very challenging. The drug delivery needles used in these devices are also extremely thin, with an external diameter of 0.3 mm. During the assembly process, they have to be bent while ensuring that there will be uninterrupted passage of the fluid in the final product (Figure 2).
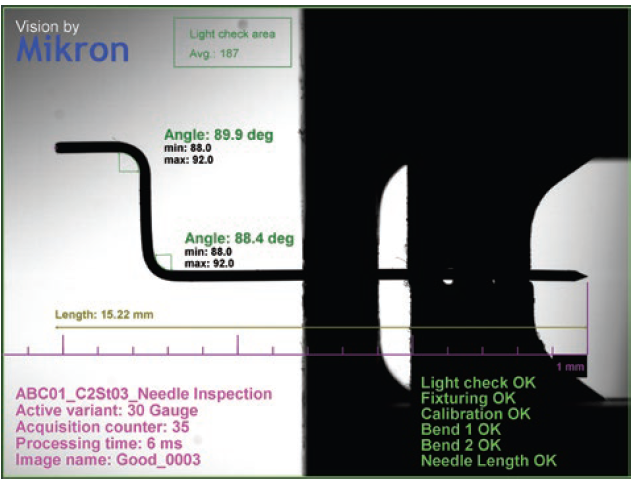
Figure 2: Highly precise control is needed to bend the needle during assembly without blocking the fluid path.
The challenge for Mikron Automation was to develop a system that could assemble these various miniature components, some not even visible to the naked eye. “Originally, our automation systems were not deployed to assemble intricate assemblies of this size and precision,” notes Mr Pointet. “We had to perform micro-assembly and micro-adjustment operations that we had never done before!”
Therefore, to meet the exceedingly high-precision requirements for the design and adjustment of these systems, the Swiss company’s engineers and technicians had to use high-resolution cameras and large screens that allowed them to zoom in on certain areas of intervention during the assembly process and make extremely fine adjustments and specific alignments (Figure 3). In the end, the technology deployed was exceptional in terms of precision and assembly efficiency.
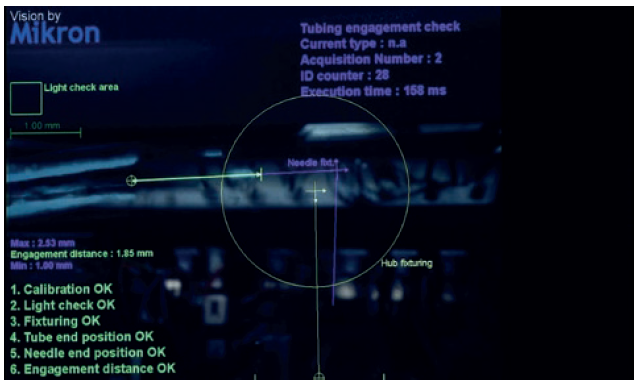
Figure 3: Precise connection between the needle and silicon tube.
SIMULTANEOUS ENGINEERING WITH THE CUSTOMER
Another challenge for Mikron in the implementation of automation in this field is that it is a rapidly developing market. Few products are currently on the market, and many are still under development. “With this type of device, our support is provided at a very early stage of the project, with products whose development is not yet finalised and that do not yet work in practice”, notes Mr Pointet. “For the past five years, clients have been asking us for help well before the clinical testing phase. They have a clear idea, prototypes of parts made in 3D printing, but they are still very far from the final product stage.”
For this reason, Mikron has expanded its pre-production services to meet some of these specific needs. To demonstrate its ability to perform certain complex operations and assemblies (Figure 4), Mikron provides proof of principles for certain assembly processes and key operations that can later be integrated into the overall assembly system. Similarly, Mikron will engage with its partners during the product development phase to suggest design improvements.
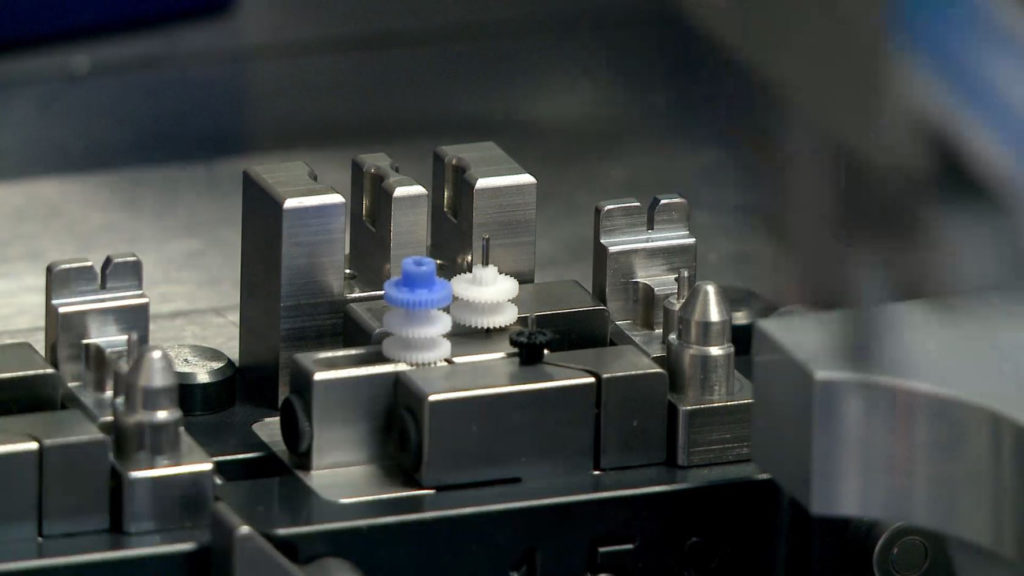
Figure 4: Assembly of micro-gears without damaging their teeth.
When performing a design for manufacturing and assembly (DFMA) analysis, Mikron will use its experience to suggest design changes that may improve the assembly process. For some projects, Mikron also provides use of its ISO 13485 certification and qualified Class 7 clean room, in which device-level tests can be carried out. As a result, the customer’s device is developed simultaneously with Mikron’s assembly system.
A PARTNERSHIP BUILT ON TRUST
For a supplier such as Mikron, being involved in a customer’s project at a very early stage means that a relationship based upon trust must be established between the two parties. “For devices like on-body injectors, where we start working with customers at a very early stage of the project, it is essential to establish a real partnership with the customer in regard to the technological challenges involved and the financial investments”, says Mr Bauer. “This implies that there is an open communication and a real collaboration between the two partners. Very often, clients are developing new products that have a lot of intellectual property. Confidentiality and trust are essential to the success of the programme.” Mikron Automation has proven to be the partner of choice for assembling on-body injectors, enabling the best risk mitigation for its customers, thanks to the security offered by its process expertise, its corporate culture based on customer success, and its global scale, which enables it to support the challenges of such projects financially (Figure 5).

Figure 5: Mikron Automation’s headquarters in Boudry, Switzerland.

