To Issue 138
Citation: Limouze R, “Overcoming the Challenges of Ultra-Large-Volume Subcutaneous Infusions”. ONdrugDelivery, Issue 138 (Oct 2022), pp 20–22.
Rob Limouze looks at the obstacles facing ultra-large-volume subcutaneous infusion drug delivery and highlights how the KORU Freedom infusion system meets these challenges.
When given the choice, patients consistently prefer subcutaneous (SC) over intravenous (IV) drug delivery.1,2,3 Use of the SC route has expanded recently through devices that can deliver up to 5 mL, more than the 2.5–3.0 mL limit of autoinjectors. Innovation in ultra-large-volume SC drugs (ULV SC) – those with volumes between 5 and 50 mL and beyond – is increasing rapidly.
“KORU Freedom uses a constant pressure system that allows the flow rate to adapt to back pressure at the injection site for an optimal patient experience.”
There are unique challenges to realising the promise of ULV SC drugs. These have been identified over a decade of serving tens of thousands of immunology patients with SC immunoglobulin (SCIg), at volumes ranging from 5 to 200 mL (without hyaluronidase) and up to 600 mL (with hyaluronidase). Ten ULV SC drugs have been approved since 2010, most recently Empaveli® (Apellis Pharmaceuticals, MA, US) (pegcetacoplan) for paroxysmal nocturnal haemoglobinuria in 2021. Of the drugs using an injector, the KORU Freedom system has been specifically cleared for use with all but one and has been used in pivotal studies supporting the drug’s approval (Figure 1).
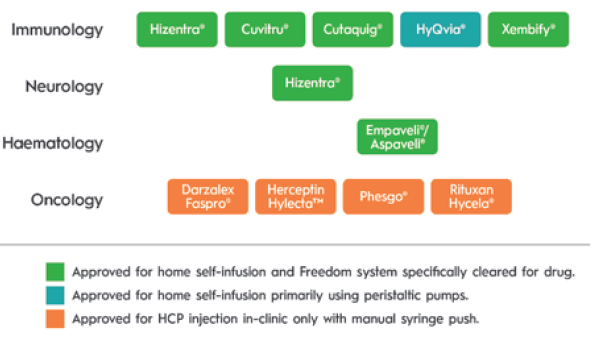
Figure 1: Drugs approved for SC injection of 5 mL or greater volume since 2005.
DEVELOPMENT AND COMMERCIAL SUCCESS DESPITE THE CHALLENGES OF ULV SC
ULV SC drug absorption differs from that of smaller volume injections. Infusion site back pressure can be higher at larger volumes and flow rates. Additionally, the impact of temperature on viscosity can have a clinically significant impact on delivery time when volumes are larger. A study of 17 immunoglobulin patients where the infusion rate slowed in response to site back pressure showed an average flow rate compensation of 20%. It also demonstrated that patients experienced variability in absorption from one infusion to the next (Table 1 & Figure 2).
| Total Number of Patients | 17 |
| Total Number of Infusions | 34 |
| Average Dose (mL) | 70.9 |
| Average Predicted Flow rate per Site (mL/Hr) | 16.8 |
| Average Measured Flow rate per Site (mL/Hr) | 13.4 |
| Average Decrease in Flow rate per Site (%) | 20% |
Table 1: Change in flow rate to adapt to injection site back pressure. (Source: KORU and third-party data on file.)
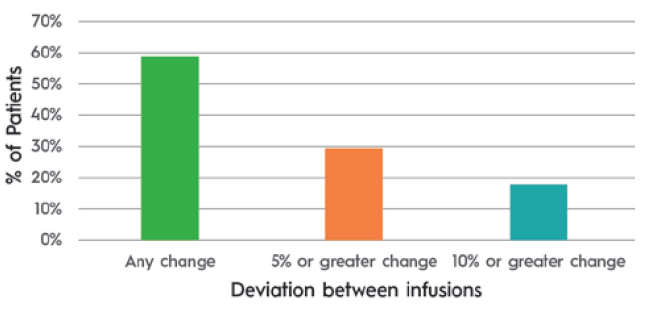
Figure 2: Patients experiencing differing flow rates between infusions 1 and 2. (Source: KORU and third-party data on file.)
At large volumes, many believe that maintaining a fixed flow rate regardless of infusion site back pressure can lead to patient discomfort. KORU Freedom uses a constant pressure system that allows the flow rate to adapt to back pressure at the injection site for an optimal patient experience.
A related challenge is accommodating the “bleb” that forms as the SC space fills with medication. The geometry and variability of bleb shape and size makes it challenging to secure a rigid patch injector throughout an entire infusion. The tethered design of the KORU Freedom infusion system accommodates variability in bleb shape and size while allowing patients to select easily between multiple infusions sites, such as legs, arms or abdomen, to accommodate lifestyle and patient preference.
The use of hyaluronidase can mitigate the above challenges by improving the rate of drug absorption by temporarily degrading hyaluronic acid and opening the SC space. However, its use creates new requirements. The flow rates made possible with hyaluronidase can exceed the back pressure tolerated by electronic systems and, depending on drug infusion requirements, customisation of system components may be needed to achieve desired treatment times.
The modular design of the KORU Freedom system allows for rapid customisation and entry into the clinic while meeting drug delivery requirements. Entry into Phase I–II studies can be accomplished with minimal development, allowing for device optimisation for Phase III without creating development delays or requiring pharmaceutical developers to fork the development path into a multiple-device strategy.
ULV SC drugs require patients to be trained in person by nurses before being certified for self-administration. Providing this education works differently in each global market. This can create a substantial training burden for drug manufacturers. Working with a partner with a fully developed training capability, as well as a channel to healthcare providers in global markets, can aid in reducing this burden. The KORU Freedom system is used to treat patients globally, with thousands of healthcare providers already experienced with the system.
Most ULV SC drugs are supplied in vials, requiring users to transfer the drug to a secondary container. This process is challenging for users and may not be possible for high-viscosity drugs. The KORU Freedom system has been cleared for use with some prefilled syringes, providing pharmaceutical companies with a means to select from multiple primary containers while eliminating the drug transfer step for patients.
SELECTING A DEVICE PARTNER
While a vendor may provide a delivery device, a device partner offers support spanning drug development and commercialisation. KORU Medical Systems offers proven success in development, clinical research, global registrations, commercialisation and therapy acceptance by tens of thousands of patients. This success rests on the combined efforts of engineering, clinical, commercial, regulatory and manufacturing teams honed by real-world feedback from patients, clinicians and regulators.
REFERENCES
- Gruenemeier P, “A retrospective review of the impact of patients converting from Intravenous Immunoglobulin (IVIG) to Subcutaneous Immunoglobulin (SCIG) of patient satisfaction and total cost of care”. IgNS 2021: AB29.
- Gingele S et al, “Switch from intravenous to subcutaneous immunoglobulin IgPro20 in CIDP patients: a prospective observational study under real-world conditions”. Ther Adv Neurol Disord, 2021, Vol 14, Article 17562864211009100.
- O’Shaughnessy, J et al. “Preference for the fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection in patients with HER2-positive early breast cancer (PHranceSCa): A randomised, open-label phase II study”. Eur J Cancer, 2021, Vol 152, pp 223–232.

