To Issue 139
Citation: Simpson I, Bhandari V, “Considering the Sustainability Impact of Connected Inhalers in the Treatment of Asthma”. ONdrugDelivery, Issue 139 (Oct/Nov 2022), pp 42–48.
Iain Simpson and Vinith Bhandari consider the sustainability impact of device connectivity specific to asthma inhalers through the reduction of greenhouse gas emissions.
In recent years, there has been increasing interest in the use of electronic drug delivery devices, based on the belief that these devices can offer patients the benefits of personalisation and ease of use. Furthermore, the use of electronics enables drug delivery devices to connect to smart devices and the internet, allowing data to be shared with other stakeholders in the healthcare ecosystem. This can enable remote monitoring, especially around measuring and improving medication adherence, as well as supporting better and more integrated disease management.1
“The shift from single-use disposable devices to electronic, reusable devices can have a positive impact for both sustainability and the patient experience.”
However, at the same time, global concerns around sustainability have impacted nearly every industry, and healthcare is no exception. At first, the addition of electronics might appear counterintuitive to sustainability objectives. However, the shift from single-use disposable devices to electronic, reusable devices can have a positive impact for both sustainability and the patient experience.2
This article explores whether device connectivity specific to inhalers used to treat asthma can positively impact overall sustainability by reducing greenhouse gas (GHG) emissions, on the basis it might reduce consumption in other parts of the healthcare system.3 The work presented in this article is the result of a research project conducted by Vinith Bhandari, as part of an MPhil in Therapeutic Sciences at the University of Cambridge and supported by Phillips-Medisize, a Molex company.4
Initial research established that, while relatively limited research has been published on reducing the environmental impact of chronic disease treatments, significant work has been done on asthma, especially by the UK NHS. The data are available to understand in detail how different aspects of the treatment pathway impact the associated GHG emissions. With the introduction of smart connected inhalers, such as Digihaler® by Teva, and connected add-on sensors, such as those provided by Propeller Health (WI, US), there is an opportunity to consider how technology can support more sustainable healthcare pathways for the disease. As a result, research has focused on asthma and connected inhalers. This article also considers how the approach might be applied to other disease areas.
ASSESSING IMPACT OF CONNECTED INHALERS ON THE TREATMENT OF ASTHMA
Nearly eight million people in the UK have been diagnosed with asthma (around 12% of the total population), with about 5.4 million receiving asthma treatment, according to the British Lung Foundation, as of 2018. Annually, about 160,000 people are diagnosed with the disorder in the UK, accounting for about 2%–3% of all primary care consultations.5 Most cases of asthma (~85%) in the UK are managed in the primary care system, but complications can result in increased medication use and the need for hospital treatment, often in the form of emergency admissions. Asthma is one of the most significant drivers for the NHS in terms of cost and demand on resources. It is estimated that 46% of asthma deaths could be avoided with better routine care.6 In fact, the total number of deaths in England and Wales from asthma in 2017 was reported to be 1,320.7
Figure 1 shows a simplified pathway for the diagnosis and management of asthma. Asthma in the UK is principally managed in primary care, involving general practitioner (GP) visits and prescriptions. Patients who have difficulty in managing their disease in this way may be referred to secondary care for consultations and training. Failure to manage the disease may result in more severe exacerbations that can result in emergency and inpatient hospital care. In terms of medication, inhaled therapy is the standard treatment option for patients with asthma and chronic obstructive pulmonary disease (COPD). Pressurised metered inhalers (pMDIs) are the most commonly used inhaler type, although dry powder and soft mist devices are also in use.
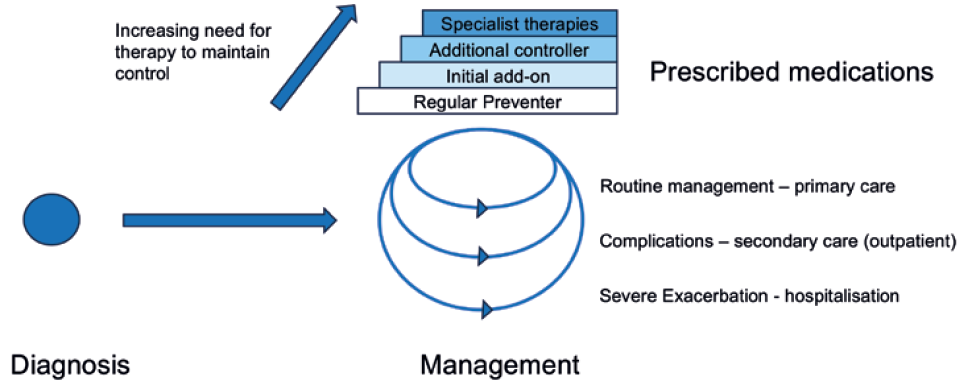
Figure 1: Simplified pathway for the diagnosis and management of asthma.
“A key objective in asthma management is to minimise the amount of medication required to achieve adequate asthma control.”
A key objective in asthma management is to minimise the amount of medication required to achieve adequate asthma control. There are two main types of inhaled medicines; preventers, which are regularly taken prophylactically to control asthma, reducing the risk of an acute attack; and relievers, which are used when a patient is experiencing asthma symptoms. A key issue in asthma disease management is that patients tend to be non-adherent to preventer use, leading to an overdependence on reliever treatments.8 As discussed later in this article, this has an adverse impact on associated GHG emissions as well as on effective disease management.
The compressed propellants, usually hydrofluorocarbons (HFCs), that are used to expel the drug from pMDIs are considered to be GHGs and account for approximately 3% of the NHS carbon footprint (and 13% within primary care).9 Chlorofluorocarbons (CFCs) have already been replaced by HFCs, but HFCs are also expected to be phased out eventually. Recent National Institute for Health and Care Excellence’s guidance recognises that sustainability should be considered when selecting which type of inhaler to use and further understands that greater use of other inhaler types could reduce emissions.10 However, there are trade-offs around cost and availability of the formulations in alternative formats that must be considered.
Another impact on associated GHG emissions is the number of hospitalisations associated with asthma. Between 2008 and 2012, there were around 60,000 admissions and a total of 200,000 bed days of care related to asthma annually, of which around 70% were considered avoidable.11 This additional care adds significantly to costs and GHG emissions and is considered an undesirable healthcare outcome.
THE ROLE OF CONNECTED HEALTH IN ASTHMA MANAGEMENT
Digital services using connected smart inhaler devices have the potential to improve the management of asthma. Results from pilot studies have shown positive outcomes. For example:
- Propeller Health reported an 18.5% reduction in the use of reliever therapy for an intervention group using its connected device technology compared with a control group.12
- In an electronic adherence monitoring study in children with asthma, a sustained improvement in adherence rates (70% compared with 49% in the control group) was seen, leading to a decrease in hospitalisations.13
- Other studies have also shown that high adherence can cause a significant reduction in hospitalisations – up to 39.5%.14,15
- Research using machine learning has shown that it is possible to predict exacerbations based on data recorded in a daily patient asthma diary.16
- Increased use of reliever therapy (something that could be monitored using smart devices) has also been associated with increased exacerbation risk.17
However, larger systematic reviews have shown less promising results, according to an analysis of several studies on adherence interventions, including patient education, reminders, simplified dosing and counselling.18,19 These reviews found that, although some interventions had a positive effect on adherence and outcomes, no single strategy demonstrated improvement in all settings. More work is certainly required to fulfil the potential of connected health in asthma treatment, but it seems likely that the introduction of optimised digital solutions can positively impact outcomes with a reduction in reliever therapies and hospitalisations.
RESEARCH METHODOLOGY
Two main steps were used in this study to consider how connected health might reduce the GHG emissions associated with asthma disease management. First, the GHG emissions associated with traditional asthma treatment in the UK were estimated. Then it was considered how a connected health intervention might influence the care pathway, which could reduce GHG emissions. To estimate the sustainability impact of asthma treatment, a model was built based on associated guidance provided by the Sustainable Healthcare Coalition (SHC).20 The model estimates the environmental impact of a care pathway in terms of GHG emissions, freshwater use (direct and indirect) and waste generation. The following care pathway modules are considered: GP visits, emergency department visits, self-management, travel by patient, and inpatient admission and bed days of care.
| Unit of measurement | GHG emissions (kg CO2e) |
Fresh water use – direct (m3) |
Fresh water use – indirect (m3) |
Waste generated (kg) |
|
| GP consultation | Per consultation | 1.10 | 0.01 | 2.27 | 0.19 |
| Patient travel Self – to GP Self – to elective care Provided – non-emergency Provided – emergency |
Per single trip |
0.56 |
0.00 |
0.10 |
0.00 |
| Emergency department | Per day | 14.00 | 0.11 | 20.80 | 0.29 |
| Inpatient admission Low intensity High intensity |
Per day |
37.90 |
0.24 |
0.56 |
3.30 |
| Self-management Patient education session |
Per session | 1.60 | – | – | – |
| Pharmaceuticals pMDIs New inhalers |
Per inhaler |
24.0 |
Table 1: The impact of parts of the care pathway and types of medication on GHG emissions.21
Using data from the SHC model, Table 1 shows the impact of parts of the care pathway and the medication GHG emissions, water usage and waste production. NHS and other published data were then used to estimate the impact of the care pathways and medication use for the UK population. These data are presented in Table 2. It was then considered how a digital intervention using a smart connected inhaler device might reduce this impact by considering two scenarios: the reduction in the use of reliever medication through digitally mediated support and the reduction in the number of hospitalisations due to better patient support and the prediction of exacerbations using digital technologies.
| Number of events/uses in UK (1,000s) |
GHG emissions (tonne CO2e) |
Fresh water use – direct (m3) |
Fresh water use – indirect (m3) |
Waste generated (tonne) |
|
| GP consultation | 75,000 | 8,250.0 | 42,000 | 17,025,000 | 1,425 |
| Patient travel: Self – to GP Self – to elective care Provided non-emergency Provided emergency |
75,000 |
42,00.0 |
0 |
750,000 |
0 |
| Emergency department | 31.2 | 436.8 | 3,432 | 648,960 | 9.0 |
| Inpatient admission: Low intensity High intensity |
93 |
3,524.7 |
22,320 |
5,635,800 |
306.9 |
| Self-management: Patient education session |
500 |
800.0 |
– |
– |
10 |
| TOTAL CARE PATHWAY | 19,433.8 | 70,848.0 | 28,783,880.0 | 1,798,684.0 | |
| Pharmaceuticals: MDIs New inhalers |
3,600 |
86,400.0 |
|||
| TOTAL PHARMACEUTICALS | 88,200.0 | ||||
| TOTAL | 107,633.8 | 70,848 | 28,783,880 | 1,798,684 |
Table 2: NHS and other published data used to estimate the impact of the care pathways and medication use for the UK population.21
RESULTS
Figure 2 shows the GHG emissions associated with the treatment of asthma in the UK. Figure 2a includes the contribution from the medication whereas Figure 2b focuses on the non-drug aspects of asthma care. As the data show, the overall impact on sustainability is dominated by the medication, which is to be expected given the high levels of pMDIs use in the UK. Pernigotti et al (2021) analysed the carbon footprint of inhalers in five different European countries, including the UK, and estimated that, by switching to switching to pMDIs using propellants with lower global warming potential or DPIs, and also considering device recycling, reductions in GHG emissions of up to 90% could be achieved.21 Much of this sustainability gain would be achieved through use of these inhalers and is more about inhaler design and supply rather than an opportunity for a connected health intervention. However, they also considered improvements in clinical practice that could reduce the usage of reliever inhalers by switching to other inhaler types and estimated that these could reduce carbon dioxide equivalent (CO2e) emissions by around 48%. In this case, a digital service that monitors inhaler usage and provides feedback and support could help this change in practice and claim some of this saving in emissions.
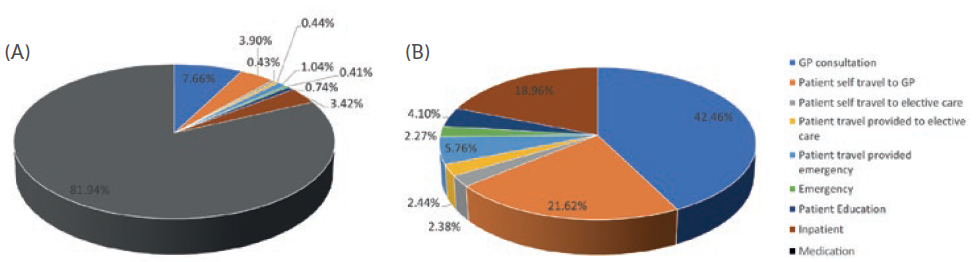
Figure 2: GHG emissions associated with the treatment asthma.
Focusing on Figure 2b and the care pathway without considering drug use, the potential to reduce hospitalisations through better disease and medication management was considered. This could be potentially mediated by a digital service that provides training support and early warning of exacerbations that might lead to hospitalisation by, for example, monitoring for increased reliever use. If it is assumed that a supporting digital service can help eliminate 60% of the potentially preventable hospitalisations, then the data in Table 3 show that around 12% of the total GHG emissions associated with the care pathway could be eliminated. Looking at other contributions, GP consultations (including associated patient travel) account for around two-thirds of the impact on the care pathway (excluding medicines). A digital service that allows remote monitoring and support could potentially reduce the carbon emissions from GP visits (both from the patient travel and possibly some of the consultation).
| Current Practice | Future Practice (with connected health intervention) |
|||
| Number of events/ uses in UK (1,000s) |
GHG emissions (tonne CO2e) |
Number of events/ uses in UK (1,000s) |
New GHG emissions (tonne CO2e) |
|
| GP consultation | 75,000 | 8,250.0 | 75,000 | 8,250.0 |
| Patient travel: Self – to GP Self – to elective care Provided – non-emergency Provided – emergency |
75,000 |
4,200.0 |
7,500.0 |
4,200.0 |
| Emergency department | 31.2 | 436.8 | 17.2 | 240.2 |
| Inpatient admission: Low intensity High intensity |
93 |
3,524.7 |
51.2 |
1,938.6 |
| Self-management: Patient education session |
500 |
800.0 |
500.0 |
800.0 |
| TOTAL CARE PATHWAY | 19,433.8 | 17,073.2 | ||
Table 3: Total GHG emissions associated with the care pathways for current practice and proposed future practice.21
Van der Kamp et al (2020) have argued that experience of asthma management during the covid-19 pandemic can support the development of multi-modal eHealth platform technologies with the possibility that it could be adapted to the needs of specific healthcare systems, providing more personalised healthcare.22 However, there have also been concerns that a lack of face-to-face contact with healthcare professionals could increase complications and a feeling of isolation, so such systems should not preclude face-to-face contact where it is preferred.
CONCLUSIONS
This study has demonstrated the opportunity for connected inhalers to have a positive impact on reducing GHG emissions in the treatment of asthma. It has also shown how they can potentially support a transition towards more sustainable medications.
There are limitations to the work: a full lifecycle analysis to consider the increased impact of a smart inhaler due to on-board electronics and off-device systems required to provide a connected health service was not conducted. However, previous work on the sustainability of smart autoinjectors suggests that these impacts can be reduced by a move from disposable to reusable devices.1 Although inhalers tend to be multi-use, fully disposable devices, the industry is now seeing alternatives, such as Boehringer Ingelheim‘s Respimat® soft mist inhaler, offering versions that are refillable, along with devices such as Turbuhaler®, marketed by AstraZeneca, that allow the connectivity module to be transferred between disposable devices. Although further research would need to be conducted to fully demonstrate this hypothesis, the impact of the device contribution can be minimised compared with the potential benefits that it can offer.
No consideration was given to the ability of a companion connected health service to bring about the behaviour changes required to avoid excessive use of reliever medicine or to take action before a loss of treatment control leads to hospitalisation. But progress in these areas is being made and, eventually, the promising results from small studies will be scaled to larger real world populations.
In terms of the sustainability of drug-based interventions, asthma may be an outlier due to the GHG emissions associated with the pMDIs that are commonly used to treat the condition, resulting in a drug delivery system contribution that accounts for more than 80% of the total emissions associated with its treatment. Other than some telemedicine publications, little published data was found around the use of connected health to improve the sustainability of other disease treatment practices. Although GHG emissions associated with other medications are expected to be much lower, cardiovascular and psychiatric diseases, such as schizophrenia, are good examples where poor medication adherence can result in lengthy hospitalisations. As such, it is expected that the use of connected health can be extended to address both sustainability and healthcare outcomes. Fortunately, in many situations, better sustainability will be correlated with better healthcare outcomes so that their value to society is aligned and additive. It is also worth considering that digital therapeutics can avoid the use of chemicals to make prescription drugs, so this factor might also have a positive impact on sustainability.
It is hoped that this research will motivate others to explore in more detail how connected health can contribute to lower GHG emissions, as well as improving healthcare efficiency, patient experience and clinical outcomes.
REFERENCES
- Simpson I, “How Connectivity Adds Value to Injectable Drug Delivery Devices”. ONdrugDelivery, Issue 101 (Oct 2019), pp 60–64.
- Fraenkel E, Sørensen B, “Sustainability with the Aria Autoinjector: a Lifecycle Assessment”. ONdrugDelivery, Issue 126 (Oct/Nov 2021), pp 36–44.
- Holmner A et al, “Carbon footprint of telemedicine solutions–unexplored opportunity for reducing carbon emissions in the health sector”. PLoS One, 2014, Vol 9(9), Article e105040.
- Bhandari V, “Role of connected drug delivery devices in healthcare sustainability”. Dissertation submitted in partial fulfilment of the degree of Master of Philosophy, University of Cambridge (July 2020).
- “What is the prevalence of asthma?”. NICE, Apr 2022.
- Mukherjee M et al, “The epidemiology, healthcare and societal burden and costs of asthma in the UK and its member nations: analyses of standalone and linked national databases”. BMC Med, 2016, Vol 14(1), p 113.
- “Asthma deaths in England and Wales are the highest this century”. Asthma + Lung UK webpage, Accessed Oct 2022.
- Reddel HK et al, “Risks associated with managing asthma without a preventer: urgent healthcare, poor asthma control and over-the-counter reliever use in a cross-sectional population survey”. BMJ Open, 2017, Vol 7(9), Article e016688.
- Tennison I et al, “Health care’s response to climate change: a carbon footprint assessment of the NHS in England”. Lancet Planet Health, 2021 Vol 5(2), p e84-e92.
- “Patient decision aid Inhalers for asthma”. NICE, Sep 2022.
- Fleetcroft R et al, “Emergency hospital admissions for asthma and access to primary care: cross-sectional analysis”. Br J Gen Pract, 2016, Vol 66(650), Article e640-6.
- Merchant RK, Inamdar R, Quade RC,” Effectiveness of population health management using the propeller health asthma platform: a randomized clinical trial”. J Allergy Clin Immunol Pract, 2016, Vol 4(3), pp 455–463.
- Morton RW et al, “STAAR: a randomised controlled trial of electronic adherence monitoring with reminder alarms and feedback to improve clinical outcomes for children with asthma”. Thorax, 2017, Vol 72(4), pp 347–354.
- Trivedi M et al, “School nurse asthma program reduces healthcare utilization in children with persistent asthma”. J Asthma, 2018, Vol 55(10), pp 1131–1137.
- George M, Bender B, “New insights to improve treatment adherence in asthma and COPD”. Patient Prefer Adherence, 2019, Vol 13, pp 1325–1334.
- Finkelstein J, Jeong IC,”Machine learning approaches to personalize early prediction of asthma exacerbations”. Ann N Y Acad Sci, 2017, Vol 1387(1), pp 153–165.
- Quint JK et al,”Short-Acting Beta-2-Agonist Exposure and Severe Asthma Exacerbations: SABINA Findings From Europe and North America.” J Allergy Clin Immunol Pract, 2022, Vol 10(9), pp 2297–2309.e10.
- Normansell R, Kew KM, Stovold E, “Interventions to improve adherence to inhaled steroids for asthma”. Cochrane Database Syst Rev, 2017, Vol 4(4), p CD012226.
- Chan A et al, “Digital interventions to improve adherence to maintenance medication in asthma”. Cochrane Database Syst Rev, 2022, Vol 6(6), p CD013030.
- “Care Pathway Carbon Calculator”. Sustainable Healthcare Coalition webpage, accessed Oct 2022.
- Pernigotti D et al, “Reducing carbon footprint of inhalers: analysis of climate and clinical implications of different scenarios in five European countries”. BMJ Open Respir Res, 2021, Vol 8(1), p e001071.
- van der Kamp MR et al, “COVID-19: Technology-Supported Remote Assessment of Pediatric Asthma at Home”. Front Pediatr, 2020 Vol 8(8), p 52.

