Citation: Fraenkel E, Sørensen B, “Sustainability with the Aria Autoinjector: a Lifecycle Assessment”. ONdrugDelivery, Issue 126 (Oct/Nov 2021), pp 36–44.
Emil Fraenkel and Bjarne Sørensen consider the sustainability of re-useable and disposable autoinjectors, its implications in adding connectivity to drug delivery devices and how it has been an important consideration for Phillips-Medisize in the development of the Aria re-usable autoinjector.
This article is a continuation of the case put forward by Phillips-Medisize’s Iain Simpson in his October 2020 ONdrugDelivery article.
INTRODUCTION
“A significant portion of the medical device industry generates the bulk of its revenue from the sale of disposable products or components, including finished autoinjector devices and their associated components.”
In September 2019, Health Care Without Harm (HCWH) published a report that estimated that the global climate footprint for healthcare is equivalent to 4.4% of global net emissions (2 gigatons of CO2 equivalent based on 2014 data from HCWH).1 To put these numbers into perspective, the healthcare industry produces twice the level of greenhouse gas emissions compared with the aviation industry.2 Recognising this impact, the healthcare industry is following other industries in developing and deploying sustainability initiatives throughout the value chain. To date, much of this work has focused on sustainability initiatives aimed towards optimising the manufacture of drug product, such as using less energy and water, but often ignored the total impact of each product and supply chain on overall sustainability.
Looking at just one part of the supply chain or product lifecycle in isolation, and only measuring a few of the environmental problems associated with it, is simply not sufficient. However, leading pharmaceutical companies now have well-defined strategies for product stewardship and environmental impact reduction, setting deadlines as aggressive as 2030 for achieving their sustainability targets – improving not only sustainability of their own operations but demanding that all parties throughout their value chain do so as well. Achieving sustainability is challenging, especially for an industry where plastics make up approximately 85% of medical equipment, and approximately 90% of medical device waste consists of disposable, one-time-use products or components.3
THE CASE FOR RE-USABILITY IN HEALTHCARE
The HCWH report aligns its findings with the Greenhouse Gas Protocol (GHGP), categorising healthcare emissions into three groups or “scopes”:4
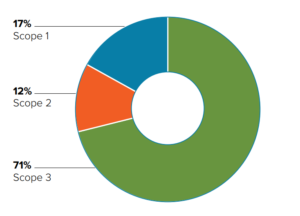
Figure 1: Classification of GHG emissions in healthcare.
- Direct emissions from healthcare facilities
- Indirect emissions from purchased energy
- All other indirect emissions that occur in the value chain, including both upstream and downstream emissions.
“The impact of product and process design on greenhouse gas emissions can be reduced if products are made usable for longer periods.”
Overall, the paper found that fossil fuel consumption is at the heart of healthcare’s emissions due to it being integral to the energy supply, raw materials, manufacture and transport of healthcare operations. Figure 1 showcases the findings; 17% of healthcare emissions are produced on site (Scope 1), 12% come from purchased energy (Scope 2) and 71% come from indirect emissions (Scope 3) – predominantly from the global supply chain involved in the production, transport and disposal of goods and services, including medical devices and instruments. As a result, manufacturers of medical devices and instruments are coming under increased scrutiny from healthcare providers and pharmaceutical companies looking to achieve better sustainability across their value chains.
A significant portion of the medical device industry generates the bulk of its revenue from the sale of disposable products or components, including finished autoinjector devices and their associated components. This business model has proved to be particularly attractive as it decreases the risks associated with contamination and inappropriate re-use, as well as the high costs associated with product reprocessing and sterilisation.
In the highly regulated medical device industry, many manufacturers see the demands of sustainable design as yet another unwelcome design restriction. Engineers and designers need to focus on compliance with strict regulatory guidelines and meeting intense time-to-market pressures. Some perceive the need for sustainability as hampering material choice and impeding innovation. The possibility of legal liability and lengthy product development cycles has also slowed the adoption of sustainable practices in the medical device industry.
Many devices, particularly invasive ones, will almost certainly continue to have a disposable component to comply with safety regulations. However, taking a new approach to the design process could have a significant and positive impact on the environmental sustainability of medical devices, including autoinjectors – the primary focus of this article.
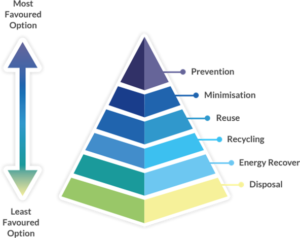
Figure 2: Waste hierarchy highlighting re-use and recycling over energy recovery (incineration) and disposal.5
Waste management is one of the biggest challenges facing sustainability-friendly initiatives. People tend to throw things away, instead of re-using or recycling them, leading to increased environmental impact from waste going to landfill or incineration. Or even worse, discarded products can end up outside regulated waste management systems with serious consequences for natural ecosystems and wildlife.
The impact of product and process design on greenhouse gas emissions can be reduced if products are made usable for longer periods of time (Figure 2). By creating devices that consumers can use for longer, companies can reduce the frequency at which their products are discarded. Products with increased longevity inherently lead to less pollution and lower risk of waste ending up where it can have an adverse impact on nature.
Experts in the field frequently make use of qualitative arguments to promote sustainability initiatives but, ultimately, we need to investigate and measure the actual quantitative environmental impact of the product/device. The most widely accepted method of achieving this is via a lifecycle assessment (LCA). This rest of this article will provide insights into the environmental performance of Phillips-Medisize’s Aria, a re-usable, electronic autoinjector, compared with the disposable autoinjectors that are typical to today’s drug delivery device industry.
AUTOINJECTORS
“Aria is a new smart autoinjector platform being developed by Phillips-Medisize to meet important emerging needs in the self injection market, including improved device sustainability.”
Autoinjectors are an important case for considering innovations that can improve medical device sustainability. These devices have become increasingly common in the treatment of chronic diseases, as they offer the convenience of safe self-administration in the patient’s home. With around 50 approved drug-autoinjector combination products on the market, the dominant design has become that of a disposable, spring-driven device, with manual needle insertion and removal, shield-triggered activation and passive needle protection. Although the approach has been favoured, as it has been seen to provide optimal safety, usability and convenience at an acceptable cost, changes in market needs are starting to challenge this approach. High on the list of these newly prominent needs is the need to better address sustainability.
LCA is a standardised, cradle-to-grave, analysis technique used to assess the environmental impacts associated with all the stages of a product’s lifecycle, from raw material extraction through materials processing, manufacture, distribution, use and disposal. Cold storage is added as a lifecycle stage in this study, as the drug often needs energy-intensive cooling prior to use. Figure 3 provides a pictorial depiction of how LCA is carried out in practice.
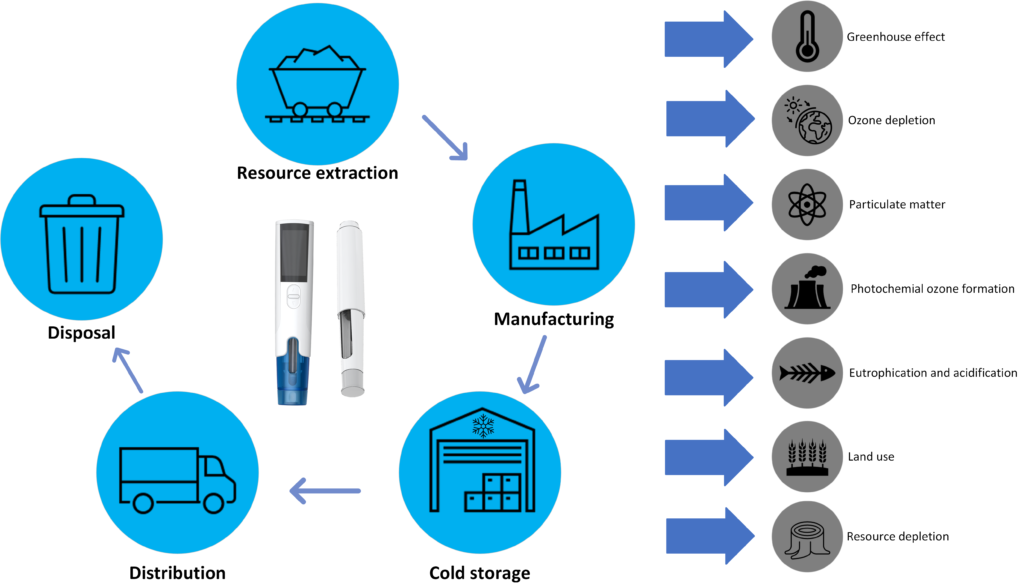
Figure 3: LCA includes analysis of impact categories standard to the strategy at each point within a device’s lifecycle.
LIFECYCLE ASSESSMENT – SCOPE OF ANALYSIS
Aria (introduced in the previous issue of ONdrugDelivery by Kate Hudson-Farmer in her article, here) is a new smart autoinjector platform being developed by Phillips-Medisize to meet important emerging needs in the self-injection market, including improved device sustainability. The autoinjector consists of a re-usable electronic power unit, which replaces the spring-powered drive in a mechanical device, coupled with a disposable cassette that contains the prefilled syringe and provides needle safety, using a moveable shield similar to most disposable devices. The cassette can accommodate both 1 and 2.25 mL prefilled syringes. There are two main models, both of which include Bluetooth connectivity:
-

Figure 4: (A) Illustration of an autoinjector with a re-usable connectivity sleeve. (B) Illustration of an autoinjector with a disposable connectivity solution.
Aria, which has a simple user interface
- Aria+, which offers several advanced features, including a graphical user interface.
The LCA method used for the study described in this article is based on the ILCD 2011 midpoint+ developed by the European Commission6,7 and the Ecoinvent 3.0 inventory database.8 The study followed relevant standards (ISO 14040 and 14044) and underwent critical review by an independent third party to ensure fair conclusions and compliance with the standards. The study focused on the Aria+ model, as it is the less sustainable of the two models, and considered both 1 and 2.25 mL cassettes. For comparison with the Aria, three common disposable autoinjectors were included in the assessment. Finally, the study also evaluated two connectivity technologies (Figure 4):
- A re-usable “add-on” sleeve
- A single-use “add-in” module that is assembled into the disposable autoinjector during manufacture and disposed of with the device after use.
The data on the Aria+ and connectivity add-ons were derived from Phillips-Medisize designs and data for typical disposable autoinjectors obtained from the analysis of commercially available devices to determine the material composition, and then interpolating information regarding manufacture, distribution, use and disposal of the devices. A limitation of the study is that the assembly process was not included for any of the devices.
Several impact categories were included within the scope of the LCA. These impact categories group different emissions into one overarching environmental effect. Each impact category was assessed throughout the LCA for each design type, allowing an apples-to apples comparison of the device design. This study focused on the following impact categories, as they were considered to have the greatest influence on environmental sustainability for the overall device design:
- Greenhouse effect
- Particulate emissions
- Ozone depletion
- Photochemical ozone formation
- Acidification
- Marine eutrophication
- Freshwater eutrophication
- Depletion of abiotic materials
- Land use.
WASTE PER INJECTION
Figure 5 illustrates the material composition of the different devices evaluated in this LCA. Most autoinjectors use a prefilled syringe with a glass barrel and steel needle, which are well characterised in terms of compatibility with drug products and hence are more difficult to replace with more sustainable materials. As such, it is more interesting to consider the use of plastic and metal in the autoinjector itself. The amount of these materials used increases with the dose size and are a key aspect of the overall device design.

Figure 5: Material composition of the Aria+ semi re-usable autoinjector, for both 1 and 2.25 mL delivery volumes, compared with three typical disposable autoinjectors. The material composition of a re-usable connectivity sleeve is compared with that of a disposable connectivity module.
Notably, the Aria cassette use.s less material than a typical disposable autoinjector. This is primarily due to Aria’s lack of a spring; naturally, not needing a spring means less metal in the cassette, but it also means less plastic, as not needing to contain a compressed spring means the cassette does not need to be as rigid as a typical disposable autoinjector. When comparing 1 and 2.25 mL systems, the impact of a semi re-usable device format is even more striking, with the plastic used increasing by 39.8%.
While these calculations provide a simple means of demonstrating waste reduction, assessing the true waste produced by a re-usable device is, in reality, more complicated. Waste reduction depends not only on materials used and product design but also on how many times the device is used throughout its operational life. Therefore, considering waste in terms of “waste per injection” is the more appropriate approach.
Waste per injection associated with a disposable autoinjector is simply the complete autoinjector, as the entire device is discarded after just one injection. On the other hand, the waste per injection for a re-usable autoinjector is defined as:
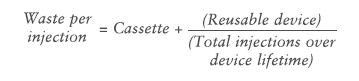
The Aria re-usable autoinjector has a specified lifetime of 550 injections and, in a best-case scenario, this limit would be reached during the intended three-year lifetime of the device. However, the study considered a more realistic base-case scenario of weekly injections over a three-year period, equating to 156 injections.
This dosing regimen was used to calculate waste per injection for the Aria+, which was then compared with that of today’s typical commercially available disposable autoinjectors. The study took packaging (secondary and tertiary) into account, as well as the instruction leaflet that would usually be provided with the device. As Aria+ is an electronic device, the analysis also included a charger and a more comprehensive user manual than the instructions for a disposable device. Figure 6 illustrates waste per injection, expressed in weight per material type.
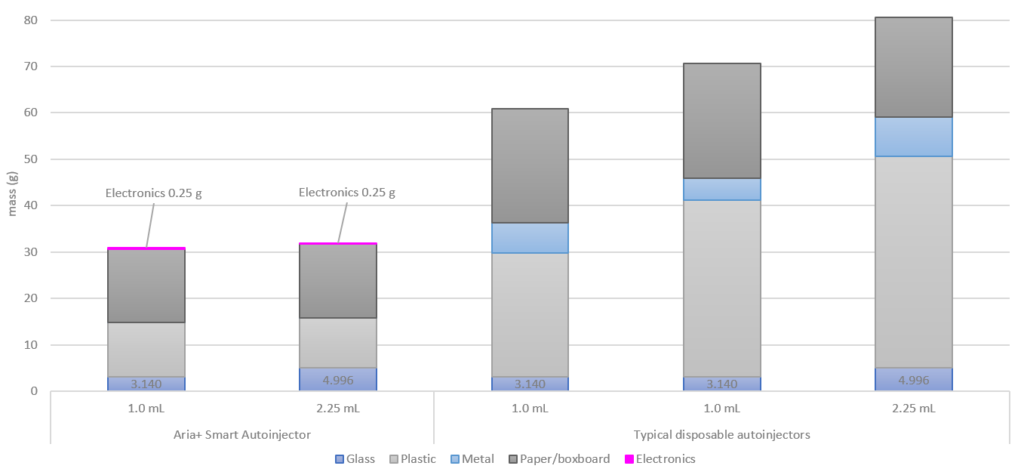
Figure 6: Waste per injection of the Aria+ semi re-usable autoinjector, compared with typical disposable autoinjectors. For the purposes of this analysis, waste per injection was determined assuming a weekly injection for three years, corresponding to a total of 156 injections.
The results highlight that about 30 g of waste is associated with the Aria re-usable autoinjector on a per injection basis, with the electronics only contributing 0.25 g per injection. Waste for the disposable autoinjectors was in the 60–70 g range for 1.0 mL devices and 80 g for 2.25 mL devices, depending on the particular commercial device considered. This represents a reduction of approximately 50–60% reduction in per injection waste for the Aria+ re-usable autoinjector, compared with typical disposable autoinjectors.
ENVIRONMENTAL IMPACT
Whereas the re-usable Aria contributes to less waste per injection than disposable autoinjectors, it does contain electronics, which have a higher environmental impact. The following results include the full lifecycle for each of the autoinjectors, including optional connectivity features, and provides a full perspective of the environmental impact in each of the principle environmental impact categories assessed.
Figure 7 illustrates the impact of the re-usable Aria compared with three typical disposable autoinjectors, also highlighting the added burden of including re-usable connectivity with the disposable autoinjectors (hashed bars). The results are illustrated as contributions to environmental impact for the disposable 2.25 mL autoinjector, which was chosen as baseline because it has the highest impact in most categories.
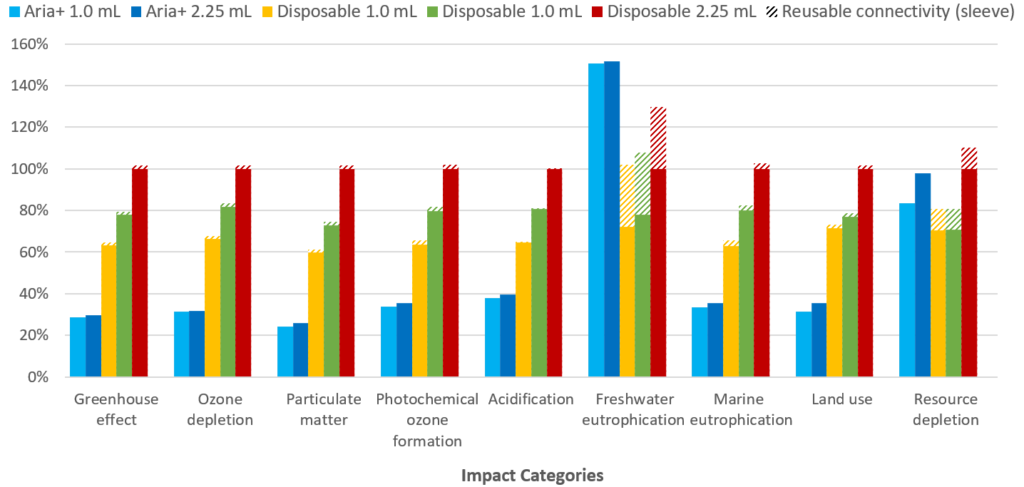
Figure 7: Percentage contributions to each of nine impact categories evaluated in the LCA. The hashed bars indicate the effect of incorporating connectivity features with a re-usable connectivity sleeve.
“From these preliminary LCA results, it can be concluded that the re- sable Aria autoinjector has the lowest overall environmental impact in the majority of impact categories and can therefore be considered a more sustainable solution than typical disposable autoinjectors, assuming a weekly injection regimen.”
The results show that the Aria autoinjector has a significantly lower environmental impact in seven out of the nine assessed impact categories. Freshwater eutrophication is higher for the Aria, compared with the disposable autoinjectors studied, due to potential sulphidic tailings (waste material remaining after ore processing) associated with the mining of metals for the electronics. The rare earth metals used in the electronic components also lead to the Aria autoinjector having a greater environmental impact than both of the 1.0 mL disposable autoinjectors for resource depletion, though it should be noted that the Aria has a lower or equivalent environmental impact to the 2.25 mL disposable autoinjector in this impact category.
For disposable autoinjectors, the inclusion of a re-usable connectivity sleeve increases the device’s impact on freshwater eutrophication and resource depletion, due to the additional materials used in the sleeve electronics. However, when a disposable connectivity module is included in the analysis, the environmental burden increases significantly. As shown in Figure 8, inclusion of a disposable connectivity module leads to a significant increase in the environmental impact in all nine categories, and up to a more than 1,300% increase in freshwater eutrophication.
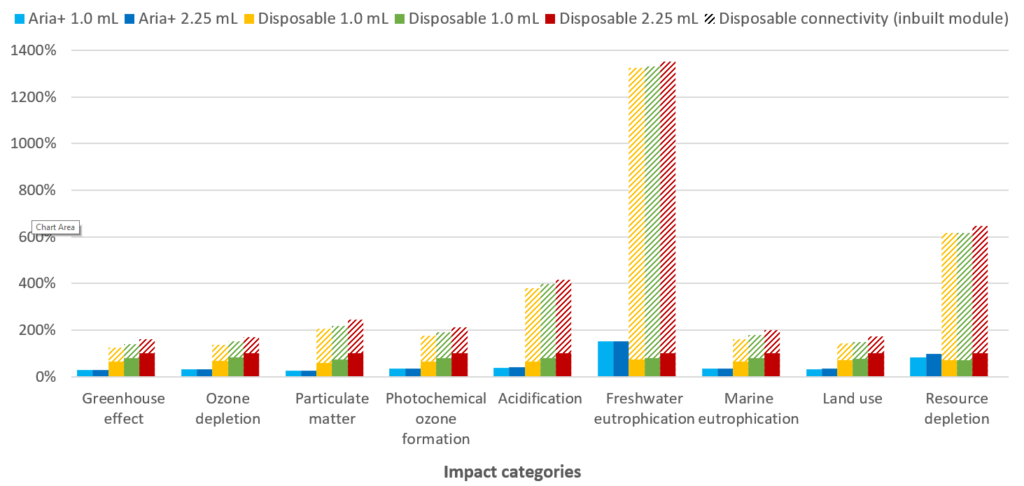
Figure 8: Percentage contributions to each of nine impact categories evaluated in the LCA. The hashed bars indicate the effect of incorporating connectivity features with a disposable connectivity module.
Disposable connectivity can therefore be seen to be challenging from a suitability perspective, compared with other available solutions. Although the add-on sleeve solution lowers the environmental impact compared with a disposable connectivity module, it requires the user to change it from one device to another, increasing user burden, meaning a user may omit this step and hence lose usage data, limiting the value of the connectivity sleeve and the connectivity features it provides.
From these preliminary LCA results, it can be concluded that the re-usable Aria autoinjector has the lowest overall environmental impact in the majority of impact categories and can therefore be considered a more sustainable solution than typical disposable autoinjectors, assuming a weekly injection regimen. Furthermore, when connectivity is added in as a device feature on disposable autoinjectors, whether a re-usable sleeve or disposable module is used, the data demonstrate that the Aria provides the best solution, from a sustainability standpoint.
Although beyond the scope of the current work, it is also worth noting that connectivity itself can also have positive impact on sustainability if it can reduce other environmental impacts, such as face-face consultations, hospitalisations and drug wastage.9
BREAKDOWN OF CONTRIBUTIONS
To further probe the environmental impact of the devices evaluated in the LCA, we can consider the cause of the emissions for each impact category. For simplicity, Figure 9 only presents the results of contributions to the greenhouse effect, also called a CO2 footprint (the full LCA study investigated all impact categories in this fashion). The results confirm an overall lower contribution to the greenhouse effect by the Aria re-usable autoinjector. The impact from device production is slightly lower when comparing the 1 mL disposable autoinjectors, but significantly lower in comparison with the 2.25 mL disposable autoinjector.
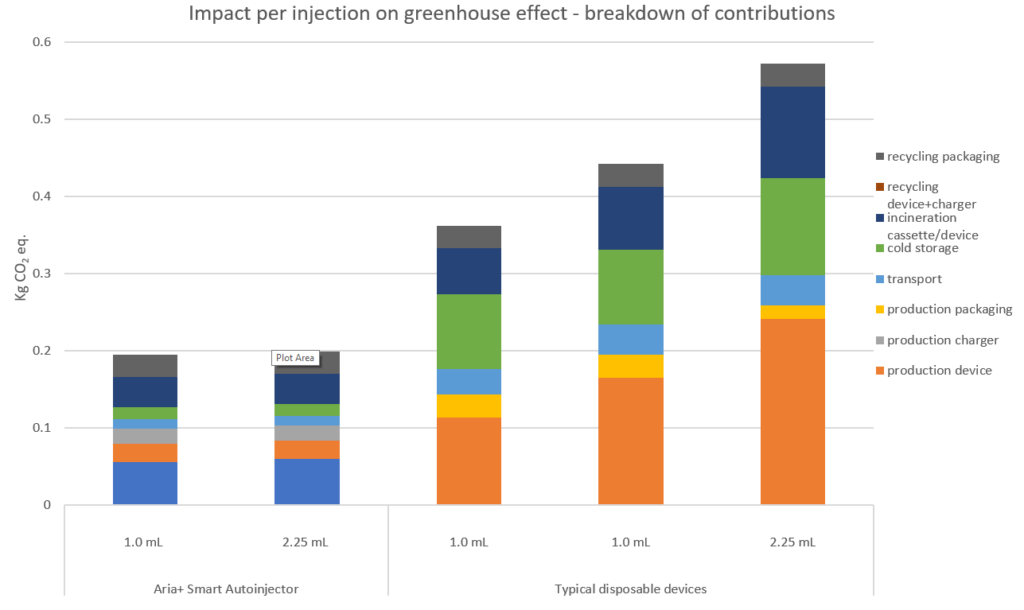
Figure 9: Per injection breakdown of contributions to the greenhouse effect for each of the devices evaluated in the LCA. As shown, drivers for the reduction of greenhouse gas emissions for the Aria+ autoinjector were savings from device production, cold storage and device/cassette incineration.
“For re-usable autoinjectors, the sustainability performance depends on how many times the device is re-used over its lifetime. By contrast, the environmental impact of disposable autoinjectors
remains fixed, because the device is always discarded after a single use.”
It is interesting to note that the Aria re-usable autoinjector has a lower contribution to the greenhouse effect for the post-production value chain activities (transport, cold storage and disposal) when compared with typical disposable autoinjectors. This is because Aria can be re-used 156 times for the weekly injections considered in the analysis, based on the study model, and therefore these contributions are split across those multiple injections. Greenhouse gas emissions due to the disposable cassette are significantly lower, as the device and cassette both use less material, weigh less and have a smaller volume, the last of which is important for cold storage.
As previously mentioned, per-injection calculations for Aria were based on a weekly injection model (156 injections over the device’s lifetime) – for re-usable autoinjectors, the sustainability performance depends on how many times the device is re-used over its lifetime.
By contrast, the environmental impact of disposable autoinjectors remains fixed because the device is always discarded after a single use. As such, to identify the most sustainable drug delivery device, pharmaceutical companies need to consider a therapy’s treatment protocols, and the total number of injections required over a relevant period. Evaluating greenhouse gas emissions, per injection, over the lifetime of a 2.25 mL Aria device, compared with a typical 2.25 mL disposable autoinjector, allows prospective users and pharmaceutical companies to see the true environmental impact of the Aria re-usable autoinjector.
Figure 10 highlights how the greenhouse gas emissions, per injection, decrease over the lifetime of a 2.25 mL Aria autoinjector. While a decrease is also observed for a typical autoinjector used with a re-usable connectivity sleeve, the emissions associated with Aria are roughly half that, even in a scenario where only monthly injections are considered. When weekly injections are considered in the model, emissions associated with Aria are less than half those of a disposable device paired with a re-usable connectivity sleeve. In contrast to devices with re-usable components, Figure 10 also shows how devices designed to be entirely disposable have a fixed per-injection emission profile – no improvement in the emission profile is observed.
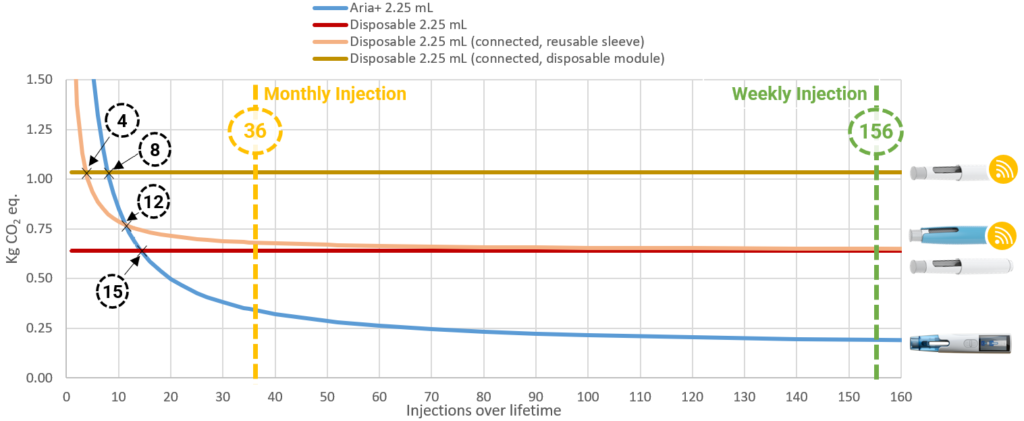
Figure 10: Correlation between injections over lifetime and impacts on the greenhouse effect for the 2.25 mL autoinjectors evaluated in the LCA.
From a treatment perspective, the results seem to suggest that if a patient only needs up to 15 injections in total, a disposable autoinjector may then be the better choice from a sustainability perspective. However, when connectivity is included, a disposable autoinjector in combination with an in-built connectivity module only has a better sustainability solution for up to four injections, and a disposable autoinjector in combination with a re-usable sleeve is better for four to eight injections. For more than 12 injections, Aria provides the most sustainable solution. Finally, considering two treatment scenarios, a monthly and a weekly injection regimen over three years, the re-usable Aria autoinjector is shown to have the lowest CO2 footprint.
CONCLUSIONS AND FUTURE WORK
It is evident from this work that the expectation of Aria as a more sustainable approach to self-administration of drugs for chronic diseases has been qualified by the use of an industry-standard lifecycle assessment. Furthermore, we have shown in other work that, in achieving this, Aria has not compromised other important requirements for autoinjectors around convenience, ease of use and safety. Pharmaceutical companies have also recognised these benefits and, importantly, so have users in the human factors studies that Phillips-Medisize has carried out.
As connectivity becomes more important in drug delivery, the fact that this can be built into re-usable electronic autoinjectors, such as Aria, creates further sustainability and/or usability advantages over similar solutions for disposable devices.
Phillips-Medisize has found the LCA approach to be very insightful in developing the device concept and design and as it progresses into clinical and commercial manufacture, and plans to repeat the calculations to support optimisation of production and distribution logistics. The company also plans to use LCA to consider more sustainable design and manufacture as a lifecycle opportunity for Aria as the availability of more sustainable materials and processes become available. Phillips-Medisize also plans to expand the work to other device platforms and programmes.
REFERENCES
- Karliner J, Slotterback S, “Health Care’s Climate Footprint – How the Health Sector Contributes to the Global Climate Crisis and Opportunities For Action”. Health Care Without Harm. Sept 2019.
- Ritchie H, Roser M, “Emissions by Sector”. Our World in Data, 2020.
- North EJ, Halden RU, “Plastics and Environmental Health: The Road Ahead”. Rev Environ Health, 2013, Vol 28(1), pp 1–8.
- “Corporate Value Chain (Scope 3) Accounting and Reporting Standard”. World Resources Institute and World Business Council for Sustainable Development, Sept 2011.
- “Waste Framework Directive”. European Commission, Nov 2011.
- “ILCD 2011 Midpoint+ is the PEF Method”. Earthshift, Oct 2016.
- “ILCD 2011 v1.0.10 Method Update in openLCA”. openLCA, Feb 2017.
- “ecoinvent LCI Database”. SimaPro website, accessed Oct 2021.
- “Digital Adherence Monitoring in Poorly Controlled Asthma – Care Pathway Case Study”. Sustainable Healthcare Coalition, Dec 2019.
BIBLIOGRAPHY
- “An Environmental Guide for the Medical Device Industry in Massachusetts”. Commonwealth of Massachusetts Executive Office of Environmental Affairs Office of Technical Assistance and Technology, Dec 2006.


