To Issue 140
Citation: Silk J, “The Influence of Uncertainty on Chemical Characterisation”. ONdrugDelivery, Issue 140 (Nov 2022), pp 92–94.
James Silk discusses the importance of accounting for uncertainty when characterising the extractables profile of a medication, with particular reference to the ISO 10993 standard.
INTRODUCTION
As is true of any measurement or experiment, analytical chemistry has uncertainties. For example, when you use the bathroom scales to weigh yourself, does the screen oscillate between two values or the arrow on the dial point between two numbers? If so, which number is correct? There is an uncertainty in the measurement of your weight to the tune of 5 g. Equally, when we say that there are 10 μg of phthalate in your sample, depending on the accuracy of the equipment and other relevant factors, we might actually be saying that it is somewhere between 9.5 μg and 10.5 μg. The ISO 10993-18 standard compels us to consider this in our analyses.
“The variation in response factors of extractables and internal standards is accounted for in the calculation of the AET.”
ISO 10993-18 – UNCERTAINTY FACTOR
The quantification of extractables is performed using screening methods, which need to be able to detect a large variety of possible extractables. The accuracy of the estimated concentrations can vary depending on the quantification method used. Quantification methods that use internal standards assume that all analytes give similar responses to each other, and therefore all respond in a similar way with respect to those internal standards. If this assumption is true, the estimated concentrations for all analytes will be very accurate. However, if this assumption is false – i.e. the response factors are not similar for all analytes – the accuracy of the estimated concentration will vary depending on the proportional difference in the response factor of the analyte to the response factor of the internal standard.
There are, however, other quantification methods that provide accurate estimates for concentrations. Calibration curves can be generated for expected extractables, using the same screening method, by injecting standards over a range of known concentrations. These will give a very accurate quantification if the same compound is found in the extracts.
Another quantification method is a hybrid of the previous two, where relative response factors are obtained for expected extractables. The relative response factors are the ratio of the standards over a range of known concentrations versus an internal standard, which produces another calibration curve. This calibration curve adjusts for the variation in response factors of extractables compared with internal standards.
The variation in response factors of extractables and internal standards is accounted for in the calculation of the analytical evaluation threshold (AET). The AET is the threshold used to determine whether a chemical detected in the test sample is of a high enough concentration to be reported. The AET is only applicable to screening methods such as gas chromatography-mass spectrometry (GC-MS) and high-performance liquid chromatography-mass spectrometry (HPLC-MS). The AET should not beused for methods designed to identify and quantify highly toxic extractables in a cohort of concern. The following formula, taken from ISO 10993-18 Annex E, is used to calculate the AET:
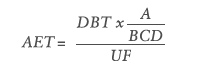
Where:
- A is the number of medical devices extracted to generate the extract
- B is the volume of the extract in mL
- C is the number of devices a patient would be exposed to in a day under normal clinical practice
- D is the concentration or dilution factor
- DBT is the dose-based threshold (such as the threshold of toxicological concern or the safety concern threshold) in μg/day
- UF is an uncertainty factor that accounts for the analytical uncertainty of the screening methods used to estimate the concentration of extractables in an extract.
“The quantification of extractables is performed using screening methods, which need to be able to detect a large variety of possible extractables.”
Each of the variables that make up the formula for calculating the AET are easily determined when preparing the extraction, except for the uncertainty factor, which must be calculated or justified beforehand. As shown by the formula for the AET, the uncertainty factor and the AET are inversely proportional to each other – a larger uncertainty factor will give a smaller analytical evaluation threshold and vice versa. A small uncertainty factor is desirable, because it shows that the variation in response factors is low and therefore suitable for reporting data, which is the foundation of a toxicological risk assessment.
For analytical methods, where the variation in response factors of the expected extractables, applied internal standards and targeted extractables using qualified methods are all known to be acceptably low, an uncertainty factor of one can be justified. An uncertainty factor of two can also be justified for screening methods that use gas chromatography-flame ionisation detection (GC-FID) or GC-MS, as the response factors of extractables detected by these methods are deemed to be somewhat consistent. For other screening methods, such as HPLC-MS, no guidance is given by ISO 10993 for a specific uncertainty factor.
However, rather than assuming and justifying the value of the uncertainty factor to be one, two or another number, the uncertainty factor can be calculated for a specific method, which gives a more accurate value of the AET and, therefore, a more reliable threshold to exclude or include peaks when reporting data to be assessed in a toxicological risk assessment for that specific analytical method. To facilitate this, ISO 10993-18 has recently had an amendment on how to determine the uncertainty factor by using the following formula, which assumes a Gaussian distribution of response factors (which is not the case for all chromatographic detection methods):
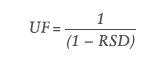
Where UF is the uncertainty factor and the RSD is the relative standard deviation of the response factors from the reference database.
“Rather than assuming and justifying the value of the uncertainty factor to be one, two or another number, the uncertainty factor can be calculated for a specific method, which gives a more accurate value of the AET.”
The reference database is an internal record of response factors specific to the analytical method that the uncertainty factor is being calculated for. These response factors are the peak areas or heights for each compound at a known concentration. One analytical method for an extractables and leachables study should have as many response factors in the reference database as there are screening methods. The RSD of a response factor can be obtained from the repeatability section of a method validation. To obtain the combined RSD for all of the compounds in the reference database, the RSDs for all of the compounds should be summed in quadrature.
The size of the uncertainty factor must not be too large or too small, as this indicates that the method being used is not suitable. A large uncertainty factor (e.g. >10) shows that the method is inaccurate and, therefore, should not be used as the basis for a toxicological risk assessment. In addition, a large uncertainty factor could give an AET that is so small that it would not be detected by the analytical method, due to it being smaller than the method’s limit of detection. If this occurs, the method should be improved before it is used as the foundation of a toxicological risk assessment.
When the RSD is greater than or equal to one, which occurs when the standard deviation is greater than or equal to the mean, the uncertainty factor will equal infinity or a negative number. An analytical method with this much variation of response factors is obviously not suitable to be used as the foundation of a toxicological risk assessment and the method should be improved.
Screening for extractables and leachables is usually done using orthogonal and complementary analytical methods, such as GC-MS and HPLC-MS. This use of multiple techniques can be used to decreasethe response factor variation and can be considered in the determination of the uncertainty factor, which is then applied to all of the complementary methods. Alternatively, a separate uncertainty factor can be calculated for each method and applied to each individual method, which gives a more accurate and specific AET than combining all of the techniques for each analytical method. Whichever is chosen, the use, value and means of calculation of the uncertainty factor used should always be justified for each analytical method used.
CONCLUSION
The purpose of chemical characterisation is to ascertain if a device is likely to be toxic or have negative effects when given to a patient, and ideally obviate the need for biological testing. The data from such an analysis is frequently used by a toxicologist to ascertain this. They will need to know how accurate the data is in relation to the AET in order to form conclusions. Here, we have shown how to quantify this as required by ISO 10993-18.

