To Issue 143
Citation: Davidson Z, “Novelia®: A Clear Vision for Eye Care for Patients Globally”. ONdrugDelivery, Issue 143 (Mar 2023), pp 24–28.
Zoë Davidson discusses the benefits of Novelia®, Nemera’s alternative to sterile filters for multidose eye droppers for preservative-free formulations.
The majority of eye drops today contain preservatives to maintain the sterility of eye drop formulations. The most used preservative is benzalkonium chloride (BAK), which has been known to damage the cornea with long-term use. Despite consistent data confirming its potential toxic effects, especially for chronic use, for example for glaucoma and dry eye disease, BAK is still used as the main preservative in eye drops.1
With the evolution of science and technology applied to ophthalmic treatments, there is an increasing need to further understand the eye, a complex organ that we all cherish and would like to protect and maintain in optimal health.2 As an industry we need to ensure that the patient is always put first.
“More than half of the bottles designed for MDPF eye drops on the market rely on a filtering system … However, there is significant research that challenges their effectiveness.”3
A SAFE PRESERVATIVE-FREE SOLUTION
There have been numerous efforts to improve the primary container closure system (CCS) of ophthalmic products. Multidose bottles have evolved to multidose preservative-free (MDPF) bottles.
To prevent the entry of bacteria into the bottle and/or to filter air, more than half of the bottles designed for MDPF eye drops on the market rely on a filtering system; 0.22 μm sterile mesh filters being the industry standard. However, there is significant research that challenges their effectiveness.3 Due to their porous structure, bacterial filters do not provide a continuous barrier to contamination.
Nemera’s alternative to the use of sterile filters for multidose eye droppers for preservative-free formulations is a non-return valve system used in conjunction with a silicone membrane to process the returning air. The non-return valve ensures that no contaminated liquid can be reintroduced to the container after the drop has been dispensed, completely removing the need to filter the liquid. The intake of air into the dispenser takes place via a separate venting system with a silicone membrane called the PureFlow™. The silicone membrane is a solid, non-porous (unlike bacterial filters) material. It is homogenous and does not contain any holes, therefore its characteristics can be precisely engineered.
In a recent paper reviewing available containers for MDPF eye drops, Campolo, Crary and Shannon stated that Novelia® has “the largest amount of published information regarding the safety and sterility of these MDPF packages … and is able to withstand both the likely microbial challenges in real-world scenarios, as well as more significant and severe challenges which could occur.”4
NOVELIA®: GIVING BACK CONTROL TO PATIENTS
In 2020, a study was conducted at Nemera’s R&D department, Insight Innovation Center, to understand these “challenges in real-world scenarios” encountered by dry eye and glaucoma patients with their current medication.5 A total of 16 participants were in the study included patients under treatment, ophthalmologists and nurse practitioners forming the healthcare provider (HCP) sample. Participants were asked to discuss their background and experience briefly as it related to managing their/their patients’ eye condition. This included symptoms, current therapies and treatment practices as well as relevant challenges or frustrations.
“Nearly nine out of 10 glaucoma patients are unable to instil eye drops correctly and so an easy-to-use system could contribute to improving compliance.”
Patients commonly cited lack of control as a key challenge relating to eye drop administration. Both dry eye and glaucoma patients cited difficulty controlling the number of drops delivered as the most common complaint. Patients noted that more than one drop may come out at a time or even leaked before being squeezed. This becomes even more problematic when the bottle is almost empty, as patients must squeeze harder to get the drop(s) out and feel they have even less control.
While a minor annoyance to dry eye patients, this is more impactful for glaucoma patients who are unsure if they have received the proper dose and/or are concerned about their expensive medication running out prematurely. Nearly nine out of 10 glaucoma patients are unable to instil eye drops correctly and so an easy-to-use system could contribute to improving compliance.6
Patients and HCPs alike desire a bottle that allows control over the number of drops delivered, consistently delivering a single drop with each actuation.
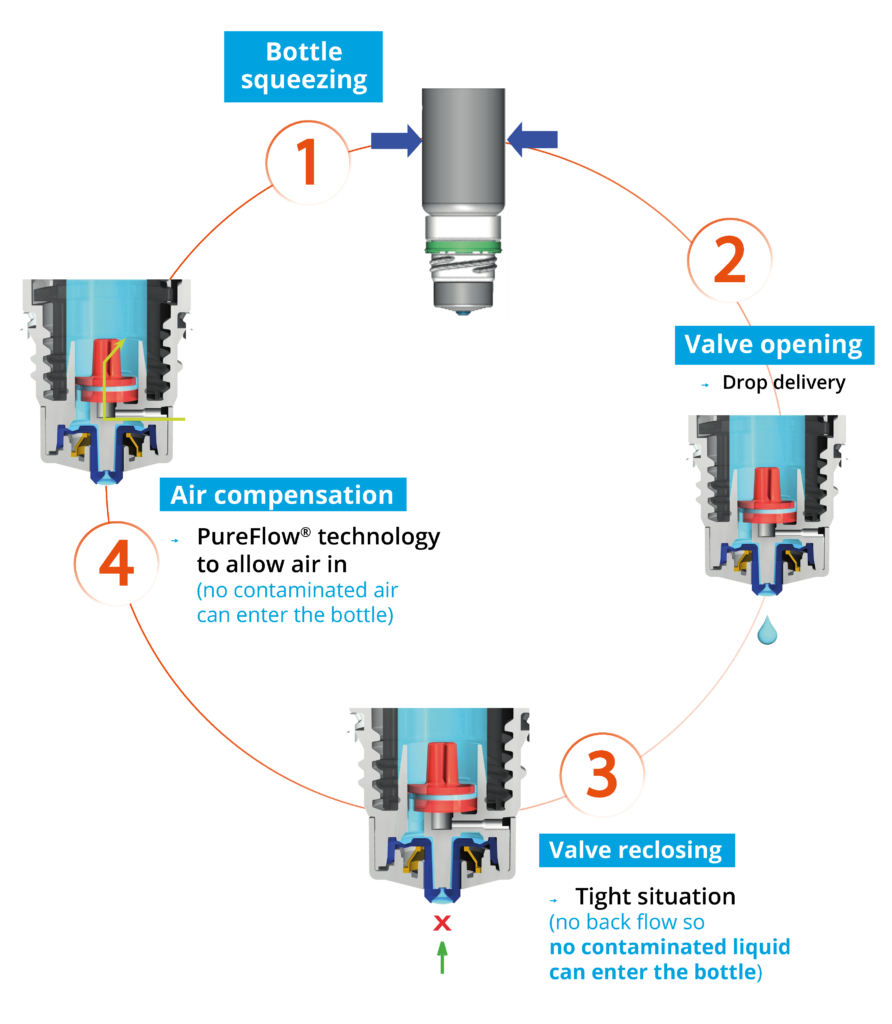
Figure 1: The Novelia® system uses a non-return valve that removes the need to filter the liquid.
“I have arthritis in my hands, and all the other brands are so hard to squeeze out even one drop. This dispenser works with gentle pressure every time. I am so glad to have found them.”
Novelia’s PureFlow™ technology not only serves as a venting system but also controls medication flow (Figure 1). Nemera has adapted the flow control within Novelia® that avoids multiple drop delivery into the eye and ensures that only one calibrated drop is dispensed at a time. Specifically, Nemera offers three different PureFlow™ versions, each tailored to formulations of differing viscosities, from highly fluid to highly viscous. In addition, five different valve sizes are available, each one delivering a different calibrated drop size. This allows Nemera’s team to customise the drop size depending on specific product requirements. This improved control leads to increased patient confidence (of accurate dosing), reduced frustration and medication waste.
“We Put Patients First” is not just a motto used by Nemera, but an attitude firmly ingrained in the company’s culture. The earlier Nemera can include patients in the development phase to assess their behaviour with a new design, the better. Patients’ needs and constraints are generally identified when defining the product design brief and are included in Nemera’s quality process and documents such as product specification, design and user failure mode and effects analysis.
EASE OF USE: THE TOPIC “IN HAND”
There is statistically significant variability in the force required to squeeze a drop from common glaucoma medications, and a representative sampling of clinic patients suggests that many likely struggle with the force requirements of several bottle designs.7
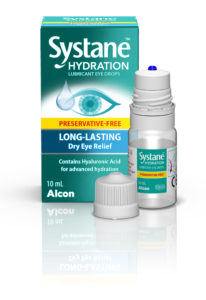
Figure 2: Systane™ Hydration PF MDPF Lubricant Eye Drops for the treatment of Dry Eyes launched in the US market in 2021 with Novelia® (image courtesy of Alcon).
In 2015, Nemera had user tests carried out by an independent company. The panel of patients interviewed fell in to two main criteria – demographics (age, gender) and type of eye condition (glaucoma, dry eye, etc.). Patients were interviewed in their own homes in both the US and the UK. These tests concluded that 76% of patients interviewed preferred Novelia® over other similar devices on the market.8
Contributing factors to the preference for Novelia® included the intuitiveness of the screw-on cap and the associated reassurance and squeeze force required towards the product’s end-of-life. Novelia® required only 6% more pressure to squeeze the bottle at the end of the treatment compared with at the beginning, whereas for other MDPFs, the increase in required pressure was 35%. Patients, even those with dexterity issues and tremors/shaking, must be able to manipulate the delivery system effectively and administer a drop.
Products on the market using the Novelia® device rate consistently high among patients, with an average star rating on Amazon of 4.7/5 (across +4000 verified customer reviews). For example, one verified Amazon US customer, in her review of Systane™ Hydration PF (Figure 2), said: “I have arthritis in my hands, and all the other brands are so hard … to squeeze out even one drop. This dispenser works with gentle pressure every time. I am so glad to have found them.”9
SUSTAINABILITY CRITERIA FOR MULTIDOSE EYEDROPPER
Patients also report concerns regarding unit doses. These include cost (as more packaging and eye drop solution per dose is required), waste and convenience, as it is easier to store a multi-use bottle in a preferred location than to ensure the patient has the correct number of unit dose pipettes with them every day.14 Handling difficulties have been noted with unit doses and their use by older patients, and inappropriate finger manipulation could be associated with an increased risk of contamination.10 Due in part to the rapidly ageing population, the number of people with glaucoma worldwide is expected to increase to over 111 million by 2040.11
In an analysis conducted by Nemera, comparing Novelia® multidose eyedropper for preservative-free formulations with unit dose packaging for a glaucoma-type regimen (one drop per eye twice per day) over one month, there was eight times less plastic used, 25 times less drug waste and nine times less energy needed for transportation for Novelia®, compared with a unit dose.12
“Today, Novelia® has more than 250 references on the market for prescription and over-the-counter products in over 55 countries across Europe, Latin America, North America, Oceania, the Middle East and Asia Pacific.”
BRINGING NOVELIA® TO PATIENTS IN CHINA
In 2022, Novelia® was successfully published on the Centre for Drug Evaluation (CDE) platform in China (Figure 3).13 The CDE performs regulatory evaluation of new drugs and medical devices to assess if they can be brought to the Chinese market. Novelia®’s publication on the CDE platform ensures that Nemera’s delivery system can be referenced through the Drug Master File (DMF) number in Drug Product Applications in China. With this first milestone achieved, Nemera customers can now submit their documentation for the evaluation of their drug products with Novelia® delivery system.

Figure 3: In June 2022, Novelia® was successfully published on the CDE platform in China.
Today, Novelia® has more than 250 references on the market for prescription and over-the-counter products in over 55 countries across Europe, Latin America, North America, Oceania, the Middle East and Asia Pacific.
A full range of low-density polyethylene (LDPE), bottles is available in 5, 7.5, 11 and 15 mL (Figure 4). Novelia®has been validated using both gamma and ethyleneoxide sterilisation. Offering two options for sterilisation allows Nemera to meet customers’ compatibility needs better. Nemera can also develop additional coloured Novelia®caps for specific demands. Finally, Nemera has developed two additional cap versions as part of the Novelia® offering to combat challenging formulations, thus expanding the scope of ophthalmic treatments served by the MDPF.
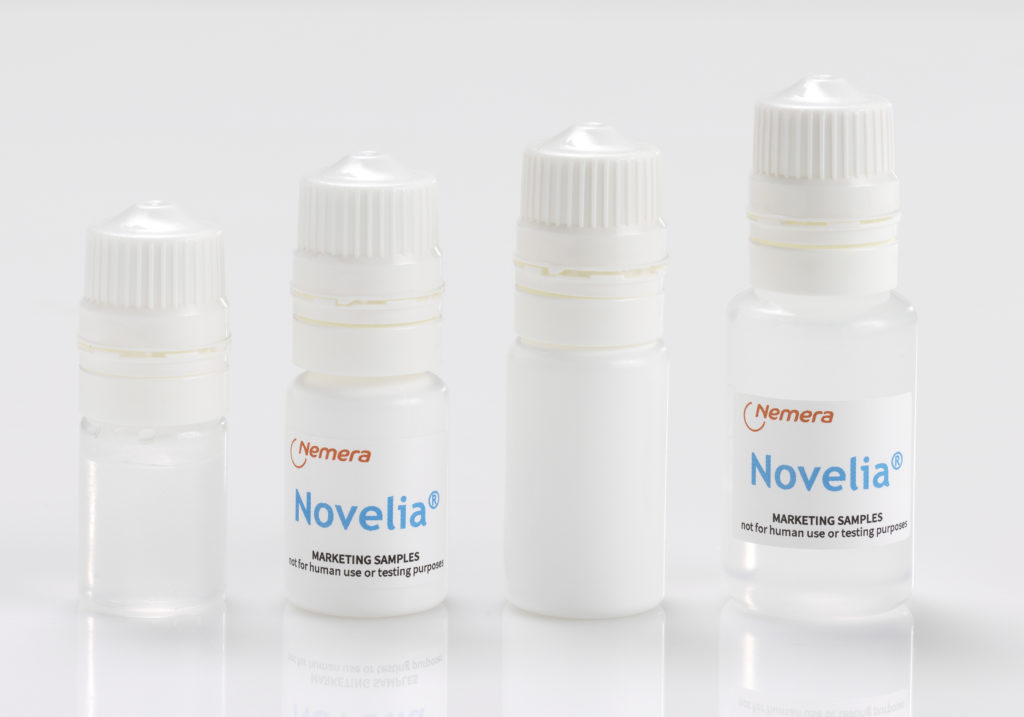
Figure 4: A full range of LDPE bottles is available in 5, 7.5, 11 and 15 mL.
NOVELIA® PRODUCTION CAPACITY EXTENSION “ACROSS THE POND”
To serve customers in supporting patient needs, Nemera is once again extending its manufacturing capabilities, this time in the US, doubling its capacity to produce the Novelia® multidose eyedropper for preservative-free formulations (Figure 5). The new >9,000 square-foot ISO 7 cleanroom, will welcome high-speed assembly lines, and numerous injection moulds and injection moulding machines. In addition, this production capacity expansion in the US has created over 35 new direct and indirect positions at the Nemera Buffalo Grove, IL, plant.

Figure 5: Nemera is currently doubling its capacity to produce Novelia® by creating two additional assembly lines in its Buffalo Grove, IL, US site.
A HOLISTIC APPROACH TO SUPPORT CUSTOMERS IN HELPING PATIENTS
Nemera offers a range of laboratory services for Novelia®, including testing of customers’ bulk formulation. Testing comprises usage simulation over two weeks, drop size analysis (variable depending on valve diameter), and flow control and squeeze force testing (beginning and near end of life). Analysis of the results allows Nemera to determine the best Novelia® configuration for a particular customer formulation. Nemera can recommend the most suitable PureFlow™ control, bottle type and valve size to achieve the desired drop calibration.
Nemera’s regulatory team is on hand to support customers with their submission filings, providing guidance on supportive documents for registration. Nemera can also assist customers in finding the right ready-to-go dossier available for private labelling certain molecules with the Novelia® delivery system. Nemera has a substantial list of partners, formulation licensors and fillers, all working in collaboration to bring to customers a finished drug-device combination product with Novelia®.
While user-friendly containers are important, educational resources that teach patients how to apply their eye drops correctly have been found to mitigate issues with unintentional noncompliance.14 Nemera can support customers with product market launch, for example, in educating sales teams and HCPs on the delivery device, dedicated training and materials to assist in promotional material creation. Customisable patient guidance videos are also available in several languages to increase patient compliance around the world.
REFERENCES
- Gupta R et al, “Evaluating eyedrop instillation technique in glaucoma patients”. J Glaucoma, 2012, Vol 21(3), pp 189–192
- Seshadri N, Uday B, “Ophthalmic Product Development, From Bench to Bedside”. Book, Springer, 2021.
- Hasegawa H et al, “Membrane filter (pore size, 0.22-0.45 μm; thickness, 150 μm) passing-through activity of Pseudomonas aeruginosa and other bacterial species with indigenous infiltration ability”. FEMS Microbiol Lett, 2003, Vol 223(1), pp 41–46.
- Campolo A, Crary M, Shannon P, “A Review of the Containers Available for Multi-Dose Preservative-Free Eye Drops”. Biomed J Sci & Tech Res, 2022, Vol 45 (1).
- “User study performed by Insight Innovation Centre”. ICH report, Chicago, USA (2020).
- Gupta R et al, “Evaluating eyedrop instillation technique in glaucoma patients”. J Glaucoma, 2012, Vol 21(3), pp 189–192.
- Moore DB et al, “Squeeze Me if You Can: Variability in Force Requirements to Extract a Drop from Common Glaucoma Bottles”. J Glaucoma, 2016 Vol 25(9), pp 780–784.
- “User study performed for Nemera by GfK to understand the Novelia® market opportunities versus competitors (Aptar OSD system)”. GfK report, Paris, France, (2015).
- US Customer Review of Systane™ Hydration PF, Company Web Page, Amazon, November 20, 2022. (https://www.amazon.com/product-reviews/B0857XD9V6, accessed February 2023.)
- Bagnis A et al, “Antiglaucoma drugs: The role of preservative-free formulations”. Saudi J Ophthalmol, 2011, Vol 25(4), pp 389–394.
- Tham Y et al, “Global Prevalence of Glaucoma and Projections of Glaucoma Burden Through 2040: A systematic review and meta-analysis”. Ophthalmology, 2014, Vol 121(11), pp 2081–2090.
- “Comparison analysis conducted by Nemera comparing Novelia® multidose eyedropper for preservative free formulations with unit dose packaging,” Internal Research, Nemera, 2020.
- “Novelia®, published on CDE (Centre for Drug Evaluation) platform in China!” Company Web Page, Nemera, October 12, 2022.
- Davis SA et al, “A randomized controlled trial of an online educational video intervention to improve glaucoma eye drop technique”. Patient Educ Couns, 2019, Vol 102(5), pp 937–943.

