To Issue 143
Citation: Wilkinson N, Wyman C, Parker M, “Beyond the Eyedropper Bottle – Three Ways to Improve Adherence”. ONdrugDelivery, Issue 143 (Mar 2023), pp 10–13.
Nathan Wilkinson, Catherine Wyman and Matt Parker discuss the future outlook of ophthalmic drug delivery beyond the traditional eyedropper bottle, including new delivery technologies, sustained-release therapeutics and digital healthcare applications.
Ever since Alcon introduced the Droptainer in 1953, the “eyedropper” bottle has been the main platform for topical delivery to the eye. Most innovation since then has centred around formulation, with advances ranging from novel pharmaceutical products to more sophisticated rheologies.
“Since Alcon introduced the Droptainer in 1953, the “eyedropper” bottle has been the main platform to offer low cost and near universal formulation compatibility.”
Enabled by this remarkably simple delivery platform, the global market for eye drops and lubricants has grown to an estimated $15 billion (£12 billion).1 It is expected to continue to grow as ageing populations suffer a greater incidence of diseases such as glaucoma and dry eye.
However, despite commercial success, this delivery modality is associated with adherence rates as low as 62%.2 This can lead to suboptimal patient outcomes for marketed products and may even mask drug efficacy during late-stage large-scale clinical studies.
Additionally, it is expected that the market will increasingly look for products that offer greater convenience and comfort for patients and that make it possible for healthcare providers to track adherence and disease progression. Digital health solutions in particular could further drive adherence if the industry develops technologies to track successful delivery to the surface of the eye and the resulting improvements in patient outcomes. The challenge is to offer these innovations at a cost point commensurate with the high-volume, low-margin markets currently still served by the eyedropper bottle.
This article looks towards the future of ophthalmic drug delivery beyond the familiar eyedropper and considers how patient outcomes can be improved through the development of products that:
- Are easier to use, even for patients with limited dexterity
- Minimise lifestyle disruption and patient burden
- Improve adherence, patient monitoring and feedback through digital and connected devices.
DEVICES SHOULD ENABLE AND EMPOWER PATIENTS TO DELIVER THEIR THERAPIES
Eyedropper bottles are difficult to use. In one study, nearly 20% of 678 participants reported difficulties getting a drop into their eye, 15% dispensed too many drops and 12% reported problems dispensing any drops at all.3 These statistics all worsen when considering patient groups with diminished dexterity, such as the elderly and young. Then, even with a successful delivery, an eyedropper administers four to five times the volume of the tear film. This results in drug wastage, the discomfort of eye drops on the cheek and a possible worsening of side-effects. All these consequences are undesirable for the patient or payer.
Products that tackle these ergonomic, aiming and dosing precision challenges are already starting to shift the industry (Figure 1). Novartis and Bausch Health (Laval, Canada) have signed agreements with device companies Kedalion Therapeutics (since acquired by Novartis) and Eyenovia (NY, US), respectively. Both companies’ devices use jetting technologies common in the printing industry to deliver fluids horizontally. While horizontal delivery is only a minor conceptual difference, it is considered to be more comfortable and convenient for patients as the user experience no longer involves tilting the head back and lifting an arm to achieve vertical alignment for drug administration.
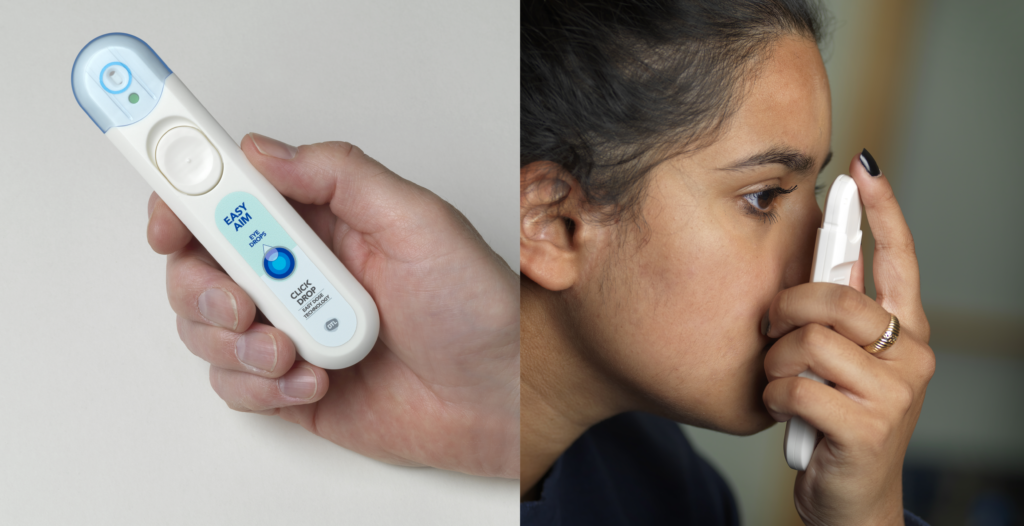
Figure 1: A device for improving usability, aiming and dose control in topical delivery. Image of CLICKDROP courtesy of Dispenser Technologies Ltd.
“Justifying the development of more complex topical delivery systems can be challenging and, at first glance, the reclassification of eye drops as combination products may seem to raise this barrier even further.”
Jetting technology also enables more precise dose control and delivery of volumes that are only one to two times that of the tear film. As well as addressing user concerns associated with overdosing, more precise dosing offers the opportunity of greater flexibility in drug loading during pharmaceutical development. Conversely, this flexibility in drug loading may come at the expense of reduced flexibility in formulation, since these jetting systems are inherently more sensitive to the rheology of the fluid compared with eyedropper bottles.
From an industry perspective, another drawback of these solutions is the increased cost and complexity the devices. The manufacturing costs of the electromechanical solutions offered by Kedalion and Eyenovia are several orders of magnitude greater than those of a standard eyedropper. Devices of this cost are only likely to be suitable for high-value therapies as companies look to preserve their margins with existing eyedroppers. However, if these devices improve adherence and patient outcomes as expected, then replicating these benefits at a lower price point will become a priority in the industry, especially for those targeting the lucrative dry eye and allergy markets traditionally served with low-cost devices.
Justifying the development of more complex topical delivery systems can be challenging and, at first glance, the reclassification of eye drops as combination products may seem to raise this barrier even further. However, the acknowledgement that the device is central to the efficacy of the therapy may present opportunities for intellectual property strategies, such as those seen in inhalation therapies. In this sphere, companies like Boehringer Ingelheim have maintained continued market exclusivity with device patents that last far longer than those of the initial pharmaceuticals. A company’s position in the market may therefore be protected through device innovation long after drug exclusivity expires. Alongside growing evidence for improved patient outcomes, this consideration may spur innovation and allow companies to justify developments that have previously been considered too high risk.
SUSTAINED RELEASE CAN ENABLE LIFESTYLES AND REDUCE PATIENT BURDEN
In addition to technologies that simplify drug delivery, sustained-release products, which deliver therapies over an extended period, are another way to improve both adherence and patient quality of life. Many therapies require eye drops to be delivered multiple times per day, which disrupts patients’ lives and, ultimately, leads to lower adherence. Technologies that enable application less frequently than once per day can lead to improved quality of life. Products that accomplish a therapeutic effect that lasts weeks or months from a single procedure can go even further towards increasing adherence.
Sustained-release products act as drug depots that slowly dissolve, erode or diffuse to release their contained therapeutics (Figure 2). Products that have been developed for sustained topical delivery can largely be categorised as devices that sit on the ocular surface but away from the cornea, such as Lacrisert (Bausch + Lomb, Canada); devices that sit near the ocular surface, such as Ocular Therapeutix’s (MA, US) Dextenza® (dexameathasone); or drug-eluting contact lenses. A persistent challenge for devices that sit on the ocular surface is that they are felt as foreign bodies. This can manifest as a physical response, such as watering or irritation, or as general discomfort. Both act as a barrier to patient acceptance. Optimising the shape of dissolvable or erodible inserts for comfort is more challenging than for contact lenses, as the rate of drug release is highly sensitive to surface area. Placement of devices on the ocular surface can also lead to loss of the insert, interference with vision and challenges with insertion.
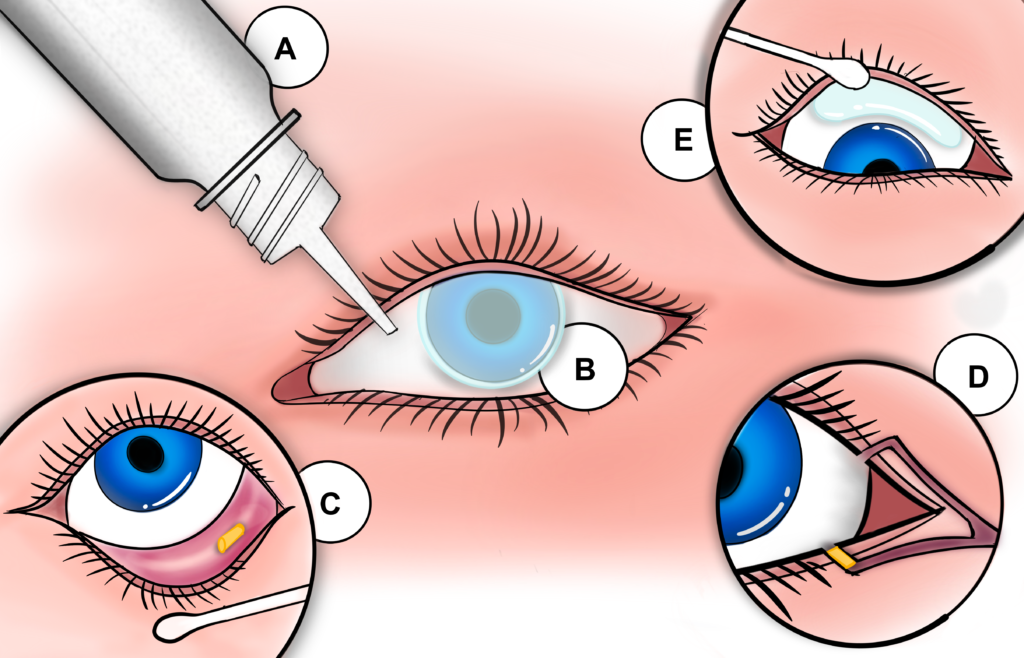
Figure 2: Example technologies for drug delivery to the ocular surface: A) Eyedropper bottle; B) Drug-eluting contact lens; C) Inferior eyelid insert; D) Lacrimal punctum sustained-release insert; E) Superior eyelid insert.
Dextenza is placed in the lacrimal punctum to deliver corticosteroids to the ocular surface for up to 30 days. This approach helps avoid the foreign body sensation but introduces new challenges as placement in the punctum forces a trade-off between the dissolution profile, drug volume and longevity of the implant. Correct placement of the device is more difficult than for devices that sit on the ocular surface and is usually done by a clinician. Understanding how devices and formulations can be designed to work within the constraints of the punctum will be essential if this approach is to be translated to chronic diseases, such as dry eye.
“If Theravision achieves commercial success, a race to develop chemistries and manufacturing processes that enable drug-eluting contact lenses in more therapeutic areas is likely to follow.”
Contact lenses are an attractive platform for sustained-release drug delivery, especially as their design has long been optimised for comfort. Johnson & Johnson’s launch of contact lenses for allergy relief, Theravison®, will be a long-awaited litmus test for the technology. If Theravision achieves commercial success, a race to develop chemistries and manufacturing processes that enable drug-eluting contact lenses in more therapeutic areas is likely to follow. However, more broadly, sustained-release inserts for topical delivery are still in their infancy. It is likely that contact lenses can teach us much about the design of inserts that can provide sustained drug delivery to the ocular surface without causing user discomfort.
IMPROVING THROUGH DIGITAL AND CONNECTED DEVICES
As with many previous waves in healthcare innovation, eye care has been at the forefront of the digital health transformation. Tools to support diagnosis are already on the market and digital therapeutics are starting to change how diseases such as amblyopia are treated. Yet, despite these successes, digital tools and connected products to support patient adherence are proving to be slow coming to market. Payers are expressing willingness to reimburse digital and connected products. However, building evidence to demonstrate their efficacy presents an interesting challenge for the eye-care industry.
For eyedroppers, there is no well-established approach for detecting successful delivery to the ocular surface, which is required for direct tracking of adherence. Most attempts at dose tracking monitor device use through detection of lid opening, changing bottle mass or ejection of a droplet. While this approach provides insight around the user’s intent to adhere, it does not capture non-adherence as a result of poor aiming. Devices like e-Novelia® by Nemera provide feedback for aiming, but no marketed drug delivery device can verify successful delivery of a therapeutic to the ocular surface. Alongside tracking a person’s success in delivering a therapeutic, digital health and connected products can play a key role in maintaining the motivation of patients to adhere to therapies.
Products that provide feedback on biomarkers related to patient outcomes could help determine the efficacy of treatments for chronic degenerative diseases. For example, a product that allows a patient to track their intraocular pressure (IOP) may drive adherence in glaucoma therapies. As an added bonus, this type of product ecosystem could also support clinical decision making by providing improved visibility of disease progression. Products are beginning to emerge that will make this approach to managing eye disease possible. Implandata (Hannover, Germany) already has a CE-marked implantable device for measuring IOP. Implandata’s approach may be attractive for those already undergoing surgery for common comorbidities, such as cataracts, but the device alone is unlikely to justify an invasive procedure. The use of contact lenses to measure IOP is gaining traction and could provide a less invasive alternative – but it remains to be seen how the performance of these devices will compare with the gold standard of tonometry. Triggerfish®, by Sensimed (Etagnières, Switzerland), is the only approved IOP-detecting contact lens device; however, its approval is based around understanding temporal variation in IOP rather than magnitude. In the short term, at-home tonometers may be capable of supporting biomarker-based feedback, but it remains to be seen whether they will be widely accepted by patients.
OUTLOOK
The challenges associated with traditional eyedroppers and the innovation discussed here signal major change for topical ophthalmic delivery. Companies are looking to introduce new topical delivery platforms as product portfolios are re-evaluated in response to long-term trends towards multidose, preservative-free packaging; changes to the EU Medical Device Regulations; and recent reclassification of eye drops as combination products. At the same time, the explosion of digital health and increased competition in consumer health is driving up patient expectations and opening new opportunities for innovators. The one-size-fits-all model is becoming outdated – products need to fit the lifestyle and needs of patients to remain competitive and deliver the best health outcomes for patients.
REFERENCES
- “Eye Drops and Lubricants Market Size, Emerging Trends and Will Generate New Growth Opportunities Status 2022 to 2030”. Press Release, Report Ocean, Jan 2023.
- Tse AP et al, “Glaucoma treatment adherence at a United Kingdom general practice”. Eye (Lond), 2016, Vol 30(8), pp 1118–1122.
- Mehuys E et al, “Eye drop technique and patient-reported problems in a real-world population of eye drop users”. Eye (Lond), 2020, Vol 34(8), pp 1392–1398.

