To Issue 145
Citation: Duband S, Chandra A, “Accelerating the Go-To-Market of Nasal Combination Products with Integrated Solutions”. ONdrugDelivery, Issue 145 (Apr 2023), pp 72–76.
Séverine Duband and Audrey Chandra discuss emerging trends in the ear, nose and throat market and how Nemera can assist in bringing new devices to market.
“With extensive experience in intranasal delivery and injectables, Nemera is developing a reliable, easy-to-use and safe nasal vaccine solution. The concept device is compatible with existing prefilled Luer lock syringes and comprises a nozzle and a dose divider.”
The overall ear, nose and throat (ENT) market was heavily impacted by the covid-19 pandemic as a result of mask-wearing, lockdowns and reduced social interaction. These factors led to reduced exposure of individuals to respiratory viruses and allergens. People also avoided going to pharmacies for non-vital medicines during the pandemic, leading to a significant 9.5% drop in over-the-counter (OTC) demand in 2020. With the return to normal life post-pandemic, there was a clear rebound in the market during 2021, with an increase of 5.7%, which required a fast, agile turnaround from the pharmaceutical industry to secure supply for patients needing medication. The ENT market is estimated at 1.9 billion devices, with the majority of drug products being for nasal applications.1
ENT MARKET TRENDS OUTLOOK
Topical therapies still dominate the nasal market for the treatment of allergic rhinitis, sinusitis and nasal congestion. Allergic rhinitis is mostly seasonal and is usually relieved by a nasal spray containing topical-acting medication. Corticosteroids are currently the first-line treatment for allergic rhinitis as they reduce swelling, inflammation and mucus secretion in the nasal cavity. This long-standing market is still very dynamic following new pipelines from the generic players, representing opportunities for growth. In fact, currently, 65% of topical treatments are generics.2
Most of these topical treatments are available OTC. In many developed countries, OTC regulations have become increasingly stringent, approaching Rx drugs dossier requirements and strengthening barriers to entry to ensure patient safety. For instance, the recent medical device regulations for the European market provide an opportunity to upgrade the required standards for raw material compliance, control strategies and product documentation, as well as for the registration of OTC drug products. This implies a similar level of stringency as that of prescription-bound solutions.
The nasal route is non-invasive and does not require intervention from healthcare professionals. Unlike injectables, patients can self-administer their medication with rapid onset. Nasal administration offers better bioavailability as it avoids the hepatic first-pass effect, which could be encountered when taking medications orally. Nasal devices are needle-free, which increases the patient acceptance level, leading to positive therapy outcomes following improved patient adherence and compliance.
More recently, there has been growing interest and further research in delivering drugs through the nose for systemically acting therapies by targeting nasal turbinates. Turbinates occupy a large surface area of the nasal mucosa and are highly vascularised, offering a convenient pathway for systemic delivery.
A rising number of prescribed systemic drugs originally administered in injectable forms have been successfully repurposed and made available as unit-dose nasal sprays. Patients and caregivers are now able to administer a one-shot spray easily and rapidly to manage emergency and crisis situations, such as overdoses, seizures and migraines. This allows therapies to target a broader patient population, increasing access to different end-user groups. To ensure patient safety, regulatory bodies impose strict regulations on this alternative route to optimise drug efficacy, requiring equivalence to their injectable counterparts, especially for life-saving drugs. The reliability of the device plays a key role in ensuring success in saving patients’ lives.
A FULL RANGE OF PUMP SOLUTIONS FOR TOPICAL, MULTIDOSE ADMINISTRATION
Nemera has extensive experience in developing and manufacturing complex nasal devices. Its journey over several decades has proven its capabilities in bringing a wide range of successful market references to market across the globe, both in regulated and low-regulated countries, to improve patients’ quality of life. Every year, Nemera’s state-of the- art GMP manufacturing facility produces millions of multidose nasal spray pumps. Its market-proven devices have been successfully commercialised for the delivery of diverse formulations, enabling its customers to offer combination products to their patients. Nemera’s expertise in spray characterisation and bioequivalence has made it possible to commercialise a wide variety of combination products (Figure 1). Today, millions of patients rely on Nemera’s devices to relieve the symptoms of chronic conditions, such as allergic rhinitis, sinusitis or nasal congestion.
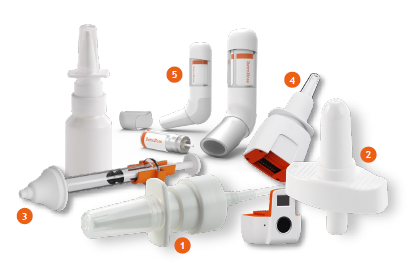
Figure 1: Nemera provides a comprehensive range of multidose pumps and offers novel technologies to tap into new market needs.
“Nemera has developed a smart electronic concept device with child-resistant, dose-counting and locking features – Safe’n’Spray, which offers solutions to prevent overdosing of potent drugs.”
SINGLE-METERED DOSE FOR SYSTEMIC DRUG ADMINISTRATION
Multidose nasal spray pumps have their place in chronic therapies, but not in acute applications. When use of the device is required to be punctual, meaning that repriming might waste valuable seconds; also, a large volume of a drug content could be wasted as it is a one-off usage. Consequently, novel therapies are starting to emerge with a precise, ready-to-use unit-dose nasal spray for emergency and crisis treatments.
Nemera’s UniSpray delivers a single, metered 100 µL dose spray and is suitable for new, repurposed or generic drugs (Figure 2). To ensure device reliability and robustness, as well as compliance with regulatory requirements, UniSpray has undergone different human factors studies, design verification and rigorous processes, assuring both patient safety and ease of use.
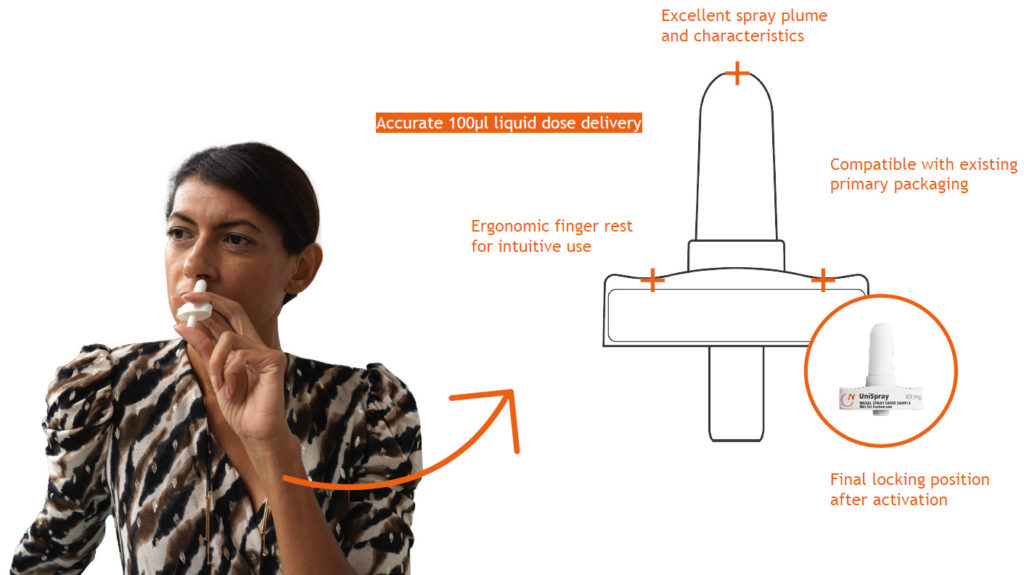
Figure 2: UniSpray, a safe and reliable single, metered spray for systemic drug administration.
Figure 3: Nemera’s spray expertise assures successful bioequivalent projects for OTC and Rx products.
This single-dose nasal spray is a ready-to-use, primeless device with 360° functionality, enabling one-handed activation. The device has an ergonomic and intuitive design to ensure its correct use. Once the device is activated, the plunger is locked in its position, preventing premature activation, which is extremely critical in emergency use, visually providing a clear message that the dose has been administered. The final locking also prevents device disassembly after activation.
UniSpray is a customisable platform, offering flexibility for spray adjustment for new formulations and generics. To accelerate time to market, UniSpray is compatible with existing marketed primary drug containers and has been adapted to fit conventional filling lines. In line with this objective, for generic drugs, the preliminary bioequivalence of selected molecules is performed by Nemera, which generates initial documentation, assuring spray characteristic equivalence and consistent performance; this foundation can then be built on by customers based on their formulation (Figure 3). For new or repurposed drugs, spray performance adjustment can also be made to ensure drug administration efficacy.
INNOVATIVE SOLUTIONS TO EXPLORE NOVEL THERAPIES
Nasal Delivery of Vaccines
Vaccine research and delivery have been accelerated amid the covid-19 pandemic. The nasal route is seen as an alternative to conventional injectable forms for vaccine delivery, benefitting from nasal-associated lymphoid tissue, a region associated with the lymphatic network, which can induce a mucosal and systemic immune response. By obtaining mucosal immunity in the nasal cavity, the immune response can combat respiratory pathogens as soon as they enter the upper respiratory tract, preventing further infection. It is also more accessible for patients with needle phobia, which is especially prevalent in younger age groups.
With extensive experience in intranasal delivery and injectables, Nemera is developing a reliable, easy-to-use and safe nasal vaccine solution. The concept device is compatible with existing prefilled Luer lock syringes and comprises a nozzle and a dose divider. It contains two doses, allowing vaccine delivery for each nostril.
Bypassing the Blood-Brain Barrier Via the Nasal Route
Another promising route under investigation is the nose-to-brain pathway, presenting opportunities to offer efficient drug delivery for therapies targeting the central nervous system (CNS). Direct-to-brain delivery would mean lower doses are required, resulting in less toxicity and fewer off-target effects, as the medications mitigate the first-pass metabolism. However, anatomically, our brain is protected by the blood-brain barrier, which prevents >95% of molecules from entering the CNS from the bloodstream. Additionally, nose-to-brain delivery poses the challenge of targeting the olfactory region of the nose, which has a small surface area and is difficult to reach.
“Not only does Nemera offer its expertise for robust device design and state-of-the-art manufacturing capacity, but it also accompanies its customers throughout the development phases with its integrated front-end solutions.”
Smart Solutions For Drug Security
Electronic components are also being explored to enhance patient safety, such as by securing potent drug administrations. Opioid treatments, such as fentanyl, are used to relieve pain with rapid onset of action through unit-dose or multidose nasal drug delivery.
Fentanyl is a potent drug, used to treat severe pain, that has become the main driver of recent increases in synthetic opioid deaths. Fentanyl is used on a regular basis by patients with cancer. For multidose nasal spray presentations, this may lead to opioid overdose when it is not used according to the treatment posology. It is therefore crucial to consider the mode and route of administration to ensure patient safety.
With this in mind, Nemera has investigated how to manage the issue of drug overdosing using a smart nasal spray device. Nemera has developed a smart electronic concept device with child-resistant, dose-counting and locking features – Safe’n’Spray – which offers solutions to prevent overdosing of potent drugs. It is an integrated device with a reusable electronic locking unit and fingerprint identification that monitors drug dose delivery in a defined period to ensure patient safety.
A HOLISTIC END-TO-END PARTNER FOR THE COMBINATION PRODUCT JOURNEY
“We put patients first” is Nemera’s bottom line principle. Its development team works actively to understand patients’ needs through discussions and tests with patients individually or in groups, as well as with round tables with key opinion leaders. Nemera’s early-stage development concepts are evaluated through user test studies to ensure a profound understanding of patients’ unmet needs. The purpose of this is, ultimately, to optimise the intuitiveness, ease-of-use and usability of the device, with the aim of reducing the occurrence of misuse and optimising the device performance when handled by patients.
Delivering drug products to the target site is challenging in the case of nasal delivery. Nemera continues to work on nasal delivery system concepts, aiming to achieve delivery to specific areas of the nasal cavity for optimum drug efficacy. To illustrate this, the company uses in vitro testing on nasal casts that replicate human anatomy to predict in vivo deposition. To support its internal projects, Nemera has developed its own nasal cast, which can also support joint development efforts with customers.
Nemera’s expertise with spray technology is also key to nasal drug delivery. Understanding the physics of atomisation and achieving good control of the spray characteristics are two of the R&D pillars for developing a nasal delivery device. Nemera actively works on design processes and tools to support its spray development and reduce the number of design iterations required.
Nemera offers design services and customised development starting from a design brief, an idea and a concept. This design phase is finalised through a validated design that can be followed by stability and clinical samples all the way down the line to industrial volumes.
Furthermore, Nemera has observed a clear growing trend in pharmaceutical companies of seeking partners who foster a holistic approach to support and help them navigate through combination product development. To this end, not only does Nemera offer its expertise for robust device design and state-of-the-art manufacturing capacity, but it also accompanies its customers throughout the development phases with its integrated front-end solutions. Nemera’s high-end laboratory facilities allow the company to offer device-plus-formulation test services, as well as the development of test methods for its customers’ needs. Finally, to navigate through a dynamic regulatory landscape, Nemera’s regulatory experts are ready to help guide projects (Figure 4).
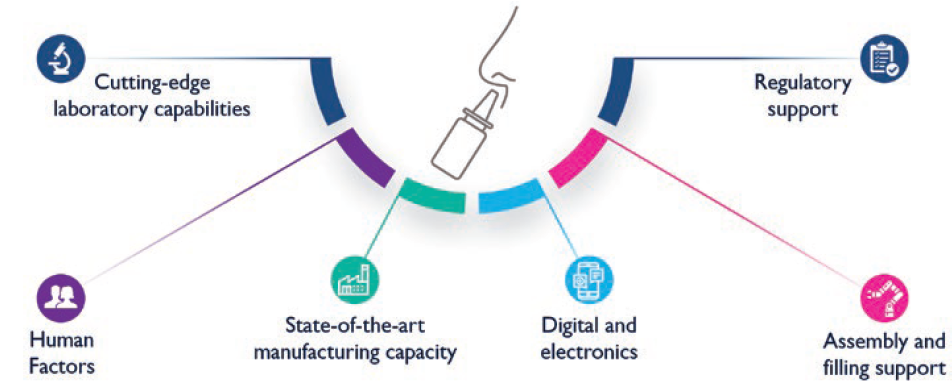
Figure 4: Full turn-key solutions to support nasal combination product development.
Following regulatory guidance is extremely crucial in establishing bioequivalence. Nemera’s in vitro testing capabilities enable it to provide comprehensive support for specific generic projects, including a complete set of tests that meet the regulatory requirements of the relevant authorities. This data generation will be statistically analysed regarding US and EU guidelines for eventual customers’ in vitro bioequivalence dossier registration filings.
In line with the fundamental idea of integrating patients within combination product development, Nemera also provides a full understanding of the patient journey and recommends user-related activities to further optimise the patient’s experience for a specific drug-device combination product. Due to Nemera’s extensive human factors capabilities, the customer could ensure that their selected device, in combination with their drug, is appropriate, safe and effective for the target population. For instance, Nemera’s support encompasses making specific instructions for use adapted to certain target populations, as well as supporting human factors activities in alignment with the customer’s chosen regulatory path, such as ANDA versus NDA.
Integrating patient insights early on in the process is critical to driving Nemera’s platform advancement. By leveraging the patient journey through its internal drug delivery device R&D, Nemera ensures that its devices meet the unmet needs of end-users and provide optimal usability and user experience.
Through capabilities in human factors engineering, user experience design, engineering, lab services, statistical expertise and regulatory support, Nemera is uniquely positioned to offer all the support that customers require through an integrated device platform and service programme.
CONCLUSION
In line with the growing interest across local-acting and systemic treatments within the nasal route, Nemera’s device platforms coupled with an integrated end-to-end approach enable the company to support and bring drug-device combination solutions to the patient. Nemera is the optimal holistic partner and helps its customers succeed in the sprint to market.
REFERENCES
- IQVIA data, 2019–2021.
- IQVIA data, 2021.

