To Issue 151
Citation: Mayer T, Bailo N, Liniger M, “Comparison of Mechatronic vs Mechanical Wearable Drug Delivery Systems”. ONdrugDelivery, Issue 151 (Sep 2023), pp 46–50.
Thomas Mayer, Nicolas Bailo and Martin Liniger highlight some of the key challenges that mechanical and mechatronic drug delivery systems must overcome when delivering large volumes of biologics subcutaneously, and explain the solutions that Sonceboz has considered during the development process of the company’s large-volume injector.
“While large bore needles or long infusion times can accomplish high-volume SC deliveries, these are not patient-preferred solutions.”
“Innovation in delivery technologies and strategies has been catalysed by the identification of unique delivery challenges associated with each class of therapeutic [small molecules, nucleic acids, peptides, proteins, and cells].” — Vargason AM, Anselmo AC, Mitragotri S (2021)1
Sonceboz is dedicated to innovation in the subcutaneous (SC) delivery of biologics, as advocated by the Subcutaneous Drug Development and Delivery Consortium.2
A unique delivery challenge for some biologics, primarily in oncology, neuroscience and rare diseases, is the ability to deliver large fluid volumes (>3 mL); such drugs are typically incompatible with the oral delivery route and require either intravenous (IV) or SC delivery. The extracellular matrix limits the volume of fluid that can be injected into the SC space. While large bore needles or long infusion times can accomplish high-volume SC deliveries, these are not patient-preferred solutions.
One solution has been to increase the concentration of the therapeutic formulation, thereby exponentially increasing the viscosity. This, too, has issues, including decreasing the chemical and physical stability of the molecule, pain on injection for the patient and a requirement for increased force or administration time.2 Pain at the injection site could reduce patient adherence to treatment.3
The invention of new on-body delivery systems (OBDSs) has been driven by the need to deliver biologic drugs subcutaneously in greater volumes or higher concentrations.4 Some of these OBDSs are powered by electronics, with reason – mechatronic systems are already found in several applications ranging from automotive, medical, aerospace, defence, robotics, manufacturing, instrumentation, Internet of Things and consumer products, and will continue to revolutionise the future (Figure 1).
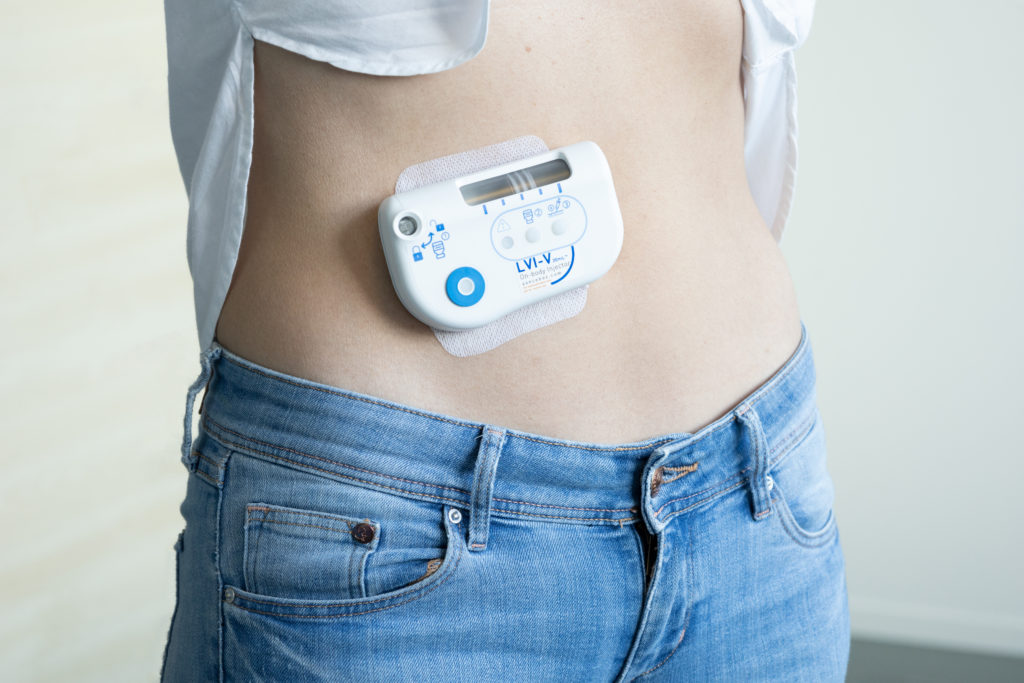
Figure 1: Sonceboz’s large-volume injector (LVI V 20 mL).
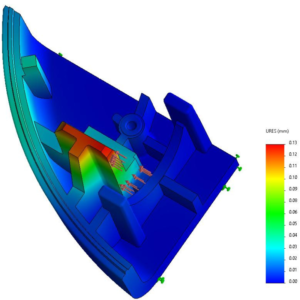
Figure 2: Design for manufacturing example with a Finite Element Method analysis to measure deformation under constraint of the end stop of a piston on the housing.
As a leading provider of mechatronic systems, with over 50 million systems manufactured and shipped across the globe each year, Sonceboz stringently follows design-for-manufacturing principles, ensuring Swiss quality for its customers in the medical and automotive industries (Figure 2). Regarding novel drug delivery systems, the company takes great care and time identifying the SC delivery challenges of biologics with mechatronic wearables. This article explains some of the identified challenges and the solutions a mechatronic OBDS could offer.
MECHATRONIC VERSUS MECHANICAL PUMP SYSTEMS
Starting with a general overview of how pump infusion systems work: fluids are transferred from the device into the patient through a pump. That pump requires an energy source that can be mechanical or electromechanical (electronic).
“In most cases, fully validated internal or even external fill-finish solutions for custom containers do not yet exist.”
In simple terms, a mechanical pump requires the movement of a structure – an actuation device – to actuate the flow of liquid. That mechanical structure can be a spring, balloon, pressurised vapour, etc. Consider how energy is transferred through a plunger in a syringe to displace the fluid within the drug reservoir.
A mechatronic pump has electrically controlled energy release, which uses an electric current to drive a motor or other motion-generating element that controls the actuation device.
DELIVERY CHALLENGE: INJECTING LARGE VOLUMES OR COMBINED THERAPIES
To reduce risk and time to market, pharmaceutical companies may prefer to use the standard drug containers (“primary containers”) that they are already accustomed to using, with the attendant fill-finish infrastructure already in place – vials, cartridges, etc – rather than using the custom primary drug containers required by some OBDS designs. Potential interactions between container materials (e.g. stoppers, closures, plungers and lubricants, such as silicone oil), coupled with the complex molecular structure of long-chain biologics, may reduce the efficacy of these drugs, which is why custom drug containers require extensive risk analyses and stability tests before regulatory approval.5 For example, silicone oil is incompatible with some biologics. In most cases, fully validated internal, or even external, fill-finish solutions for custom containers do not yet exist.
Using existing primary drug containers is the easiest, fastest and most cost-effective solution; however, clinical batches may be filled in containers that do not hold the total intended dose for clinical evaluation, especially during the early clinical phases of drug development. Multiple vials may need to be connected to the OBDS in sequence to deliver the intended dose when the dose requires a volume that exceeds the available volume of one vial. This capability is also beneficial for therapeutic regimens that use a combination of drugs.
The Sonceboz LVI-V model has both functionalities, making it highly versatile. At the heart of the company’s unique patented pump device are two electrically powered, low-noise stepper motors accurately driving two pump pistons that control the omnidirectional flow of liquid according to programmed parameters. Examples of those parameters could be:
- Flow from one drug container to another* (e.g. vial to vial, vial to cartridge, cartridge to cartridge, etc) if reconstitution is needed.
- Flow from up to two drug containers* into the patient’s SC tissue.
The two pistons work as follows: a valve piston connects to three ports successively (the connection points for the external drug containers and injection needle), selecting the ports needed for pumping. The second piston, a pump piston, generates flow by creating a vacuum in the primary drug container(s), actively drawing the liquid in a non-turbulent and gentle manner, similar to filling a syringe. The liquid is then “pushed” into another port.
Connecting to three ports is useful for loading the device with more than one primary drug container. The Sonceboz platform allows delivery volumes of 1–20 mL in its basic configuration. It also allows the delivery of highly concentrated formulations of up to 200 cPs.
That is the heart. The brain is the smart microprocessor on the circuit board, which can be programmed to ensure that the intended dose is delivered in a predefined time and deliver drugs according to patient- and drug-specific profiles.
“The Sonceboz system has been tested in multiple formative human factors trials and was found to be intuitive and easy to use for healthcare professionals and patients alike in both the USA and the EU.”
DELIVERY CHALLENGE: ENSURING THAT THE INTENDED DOSE IS DELIVERED IN A PREDEFINED TIME
A well-designed pump should reduce dead volume and ensure that the intended dose indicated on the drug label is delivered, regardless of placement orientation or injection site variation. Sonceboz’s design accomplishes these things with the following features:
- A vacuum to maximise the volume of drug drawn from the primary container
- A device that transfers the drug from the primary drug container (usually a non-collapsible container, such as a vial or cartridge) to a collapsible container (the internal reservoir with a trailing plunger), ensuring that the pump can access the drug regardless of the device’s or patient’s spatial orientations.
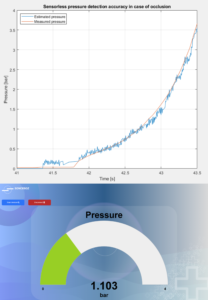
Figure 3: The large-volume injector features a sensorless pressure detection system.
Maintaining a precise and constant flow rate across the total container volume is essential to ensure that the intended dose is delivered in a predefined time window. For this, the actuation component matters. A linear spring, for example, cannot provide a constant force; as a spring becomes less compressed, the force exerted on the fluid decreases correspondingly. Theoretically, with mechanical systems, if the force of the actuation component on the liquid is not greater than the force of resistance (the back pressure) of the fluid being displaced, it is possible that the mechanical system would not deliver the total dose to the patient. Consequently, the injection time is affected by the back pressure.
With a mechatronic system, this is a non-issue. Sonceboz’s mechatronic drive system achieves extraordinary flow accuracy of ±5% and has a factory programmable injection rate of up to 3 mL/min.
Furthermore, Sonceboz has used its know-how in motor driving to incorporate a sensorless system that detects pressure on the cannula and responds by automatically decreasing the flow rate in a predefined way, as excessive tissue pressure can leadto pain and leakage at the injection site, potentially leading to an incomplete injection or loss of drug. Such a system will also detect occlusions more accurately, allowing for an appropriate device response (Figure 3).
DELIVERY CHALLENGE: DELIVERING THERAPEUTICS ACCORDING TO PATIENT- AND DRUG-SPECIFIC PROFILES
A programmable delivery device can be beneficial during the commercial phase and Phase I and II clinical trials when an investigational new drug’s optimal dose and flow rate have yet to be established.
Using near-field communication (NFC) technology to set a delivery program while the device is still in its sterile packaging allows new possibilities for use in clinical trials, as well as when patient-specific dosing is required (e.g. weight-based regimens).
With wide-ranging programming options, pharma companies can easily leverage Sonceboz’s pump across multiple drug products, as well as across a single product’s lifecycle and different patient needs.
DELIVERY CHALLENGE: AVOIDING REDESIGNS
Many OBDS designers promote the design of custom primary drug containers, which they suggest would improve the ease of use for patients. However, such custom components may require a substantial redesign of both the device and the drive system of a mechanical device whenever there is a change to the format or size of the primary container. A mechatronic device with a pump system independent of the drug container would only require software adaptations whenever the format or size of the primary container changes, as sometimes happens throughout a product’s lifecycle.
Sonceboz’s mechatronic OBDS makes it possible to connect to various existing primary drug containers of capacities up to 20 mL or even higher, if needed, such as vials or cartridges – without changing the overall system architecture of the OBDS.
DELIVERY CHALLENGE: SIMPLIFYING THE USER EXPERIENCE
In addition to NFC technology, the Sonceboz OBDS will come with built-in Bluetooth low energy connectivity, enabling the device to connect to cloud-based digital healthcare systems, thus allowing healthcare professionals to monitor adherence and provide engaging feedback to patients.
Simplifying the user experience relates to ensuring that the intended dose is delivered; mechanical systems generally have more assembly and preparation steps for the user than automatic mechatronic systems, creating more opportunities for human error. The Sonceboz system has been tested in multiple formative human factors trials and was found to be intuitive and easy to use for healthcare professionals and patients alike in both the USA and the EU.
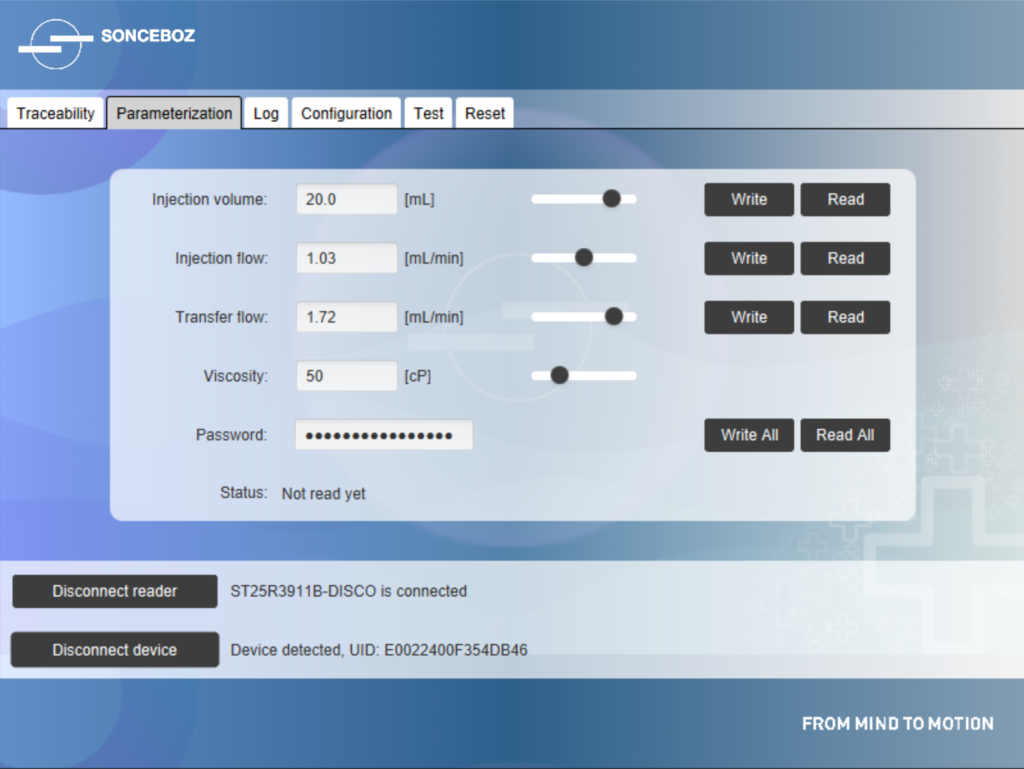
Figure 4: Near-field communication interface for parameterisation of the device’s volume and flow rate depending on drug viscosity.
Even when the battery is fully depleted after use, NFC technology allows an authorised user to collect data (Figure 4). Sonceboz pays great attention to cybersecurity issues and has implemented protective features, such as password-protected access and data encryption. Sonceboz also complies with Europe’s General Data Protection Regulation by pseudonymising all data collected from the device to protect each patient’s personal information.
DELIVERY CHALLENGES NEED SOLUTIONS
Over the past few decades, there has been much innovation in delivery technologies and strategies for all therapeutics, including biologics, which were historically only delivered by IV infusion. SC delivery continues to be preferable to IV infusion for several reasons, including:
- The patient or a caregiver can administer the treatment at home instead of visiting a clinic or hospital to have the treatment administered by a healthcare professional
- Fewer medical appointments mean treatment is less time-consuming and inconvenient for the patient, especially if therapy requires frequent administration or if the disorder is a chronic condition requiring long-term treatment
- A lower overall cost of healthcare
- Improved patient adherence
- Possibly fewer infusion-related reactions.2
While there have been many novel drug delivery systems released on the market during this time, including OBDSs, the SC delivery of large-volume (>3 mL) biologics through a simple, wearable device that can be programmed with adjustable delivery profiles without changing the drive system or drug containers has yet to become available.
Sonceboz’s goal is to provide a single multifunctional device that is tried and tested across a range of volumes and viscosities and can be tailored to its intended delivery regimen at the point of care, without the need for hardware modifications and with the ability to set selected parameters in the software.
Sonceboz’s OBDS is ready for evaluation by pharmaceutical companies. The company is here to help pharmaceutical companies and partners deliver drugs at various speeds across various volumes and viscosities while providing safe and consistent drug delivery.
* Sonceboz’s large-volume delivery platform comes in different variants: the vial-connecting injector (LVI-V), the prefilled and preassembled injector (LVI-P), the prefilled injector assembled by the user at the point of use (LVI-U), the dual-cartridge injector (DCI) and the auto-reconstitution injector (ARI). Both DCI and ARI can direct flow from one drug container to another. The LVI-V can mix two drugs within its internal reservoir. Some variants can direct flow from up to two drug containers sequentially, while the LVI-V holds both drugs in its internal reservoir and delivers the drugs simultaneously.
Find out more about Sonceboz’s large-volume delivery platform at: sonceboz.com/en/wearable-injectors.
REFERENCES
- Vargason AM, Anselmo AC, Mitragotri S, “The evolution of commercial drug delivery technologies.” Nat Biomed Eng, 2021, Vol 5(9), pp 951–967.
- Badkar AV et al, “Subcutaneous Delivery of High-Dose/Volume Biologics: Current Status and Prospect for Future Advancements.” Drug Des Devel Ther, 2021, Vol 15, pp 159–170.
- Usach I et al, “Subcutaneous Injection of Drugs: Literature Review of Factors Influencing Pain Sensation at the Injection Site.” Adv Ther, 2019, Vol36(11), pp 2986–2996.
- Oakley T, “Wearable Injectors, Latest Devices and Recent Trends.” ONdrugDelivery, Issue 111 (September 2020), pp 6–9.
- Bauert D, Kumar Busimi A, “Expert View: Primary Packaging for Wearable Injectors.” ONdrugDelivery, Issue 100 (Sep 2019), pp 26–28.

