To Issue 172
Citation: “Product Showcase: CCbio’s Injector Platform Products”. ONdrugDelivery, Issue 172 (May 2025), pp 46–48.
CCBio’s expanding portfolio of self-injection devices reflects the growing importance of at-home administration in driving adherence across a range of therapies. With reusable and disposable options spanning cartridge- and PFS-based platforms, and capabilities supporting multidose, variable dose and dual-drug delivery, CCBio’s range meets the diverse needs of patients and partners. This showcase explores the clinical rationale behind self-injection and details CCBio’s latest innovations, including electronic-assisted devices and Taiwan-based automated assembly.
THE IMPORTANCE OF SELF-INJECTION
There is clear evidence to suggest that self-injection is an effective strategy for improving adherence to medication in gastrointestinal therapies. This is largely because pen injectors, compared with traditional injections administered in a clinical setting, reduce discomfort and enhance user-friendliness (Figure 1).1
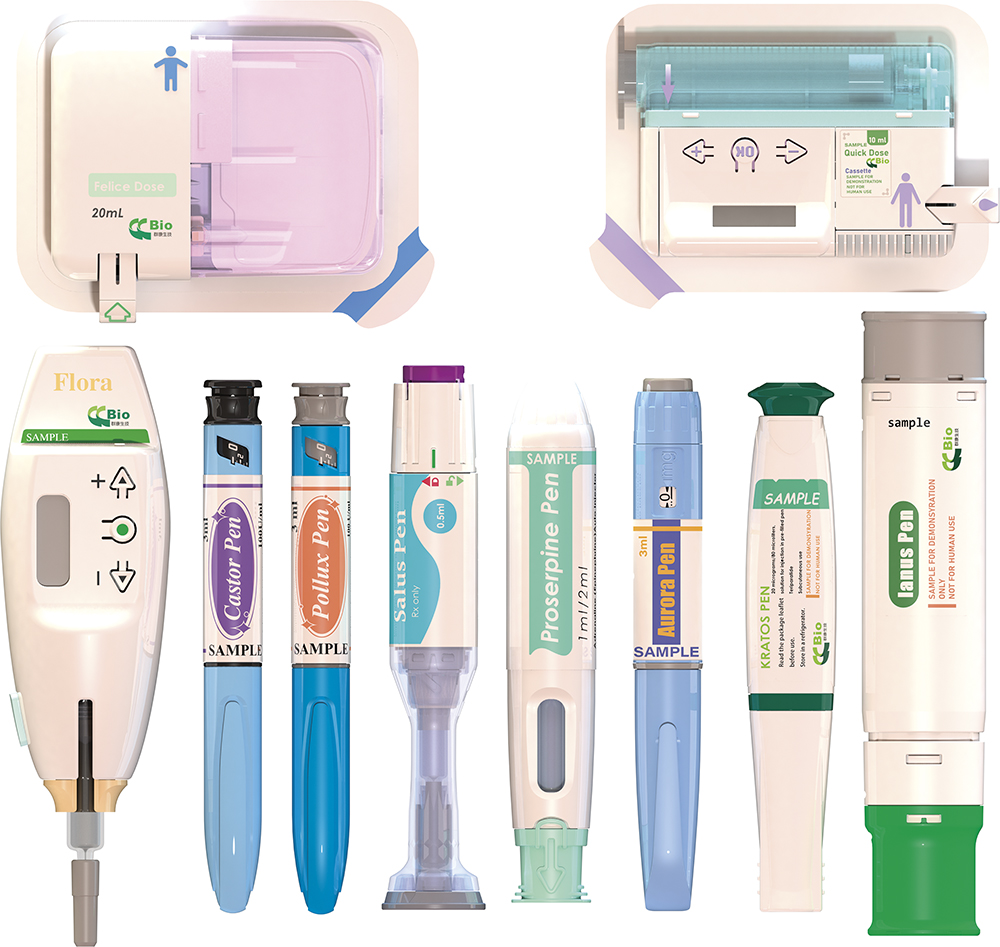
Figure 1: CCBio’s portfolio of self-injection products.
The self-injection process has evolved over time to meet the demands of a variety of medications, resulting in the development of cartridge-based designs with variable dosing and multidose capabilities, as well as designs based on single-use prefilled syringes (PFS). Medications that require frequent daily injections and make use of self-injection devices include insulin, human growth hormone, follicle-stimulating hormone, adrenaline (epinephrine), glucagon-like peptide 1 (GLP-1) and parathyroid hormone.
Future advancements will likely focus on the sustainability and reusability of self-injection pens. Reusable injection pens were first introduced in 2000, primarily targeting insulin and the diabetes market. Today, over 12 million reusable pens and more than 1.7 billion prefilled pens are sold annually, with GLP-1s driving additional growth in the demand for injection pens.
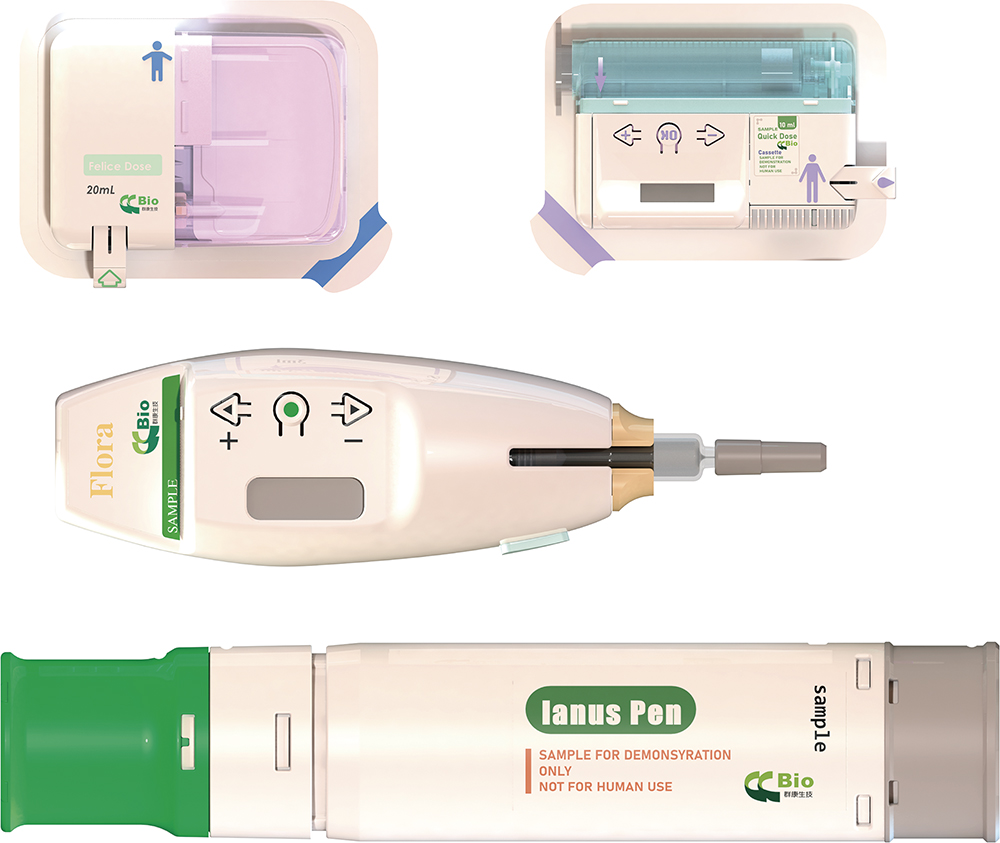
Figure 2: CCBio’s portfolio of advanced injector platform products.
CCBIO’S SELF-INJECTION PRODUCT PORTFOLIO
From initial design to practical market applications, CCBio has developed a suite of innovative injector platform products based on patient-centric usage patterns for self-injection devices (Figure 2). These injectors, both autoinjectors and on-body injectors, are designed to provide pharmaceutical partners with superior solutions, offering enhanced convenience and functionality (Table 1).
| Property | Flora | Felice Dose | Quick Dose | Ianus |
| Device Type | Electronic-assisted autoinjector |
Electronic-assisted on-body injector |
Electronic-assisted on-body injector |
Two-drug autoinjector |
| Usage | Mutidose; reusable | Single dose; disposable | Mutidose; reusable | Single dose; disposable |
| Dose Volume | 0.01–1 mL | 0.1–40 mL | 0.01–40 mL | 0.1–3 mL |
| Injection Needle | User installs PFS or cartridge with 32G needle | Hard needle: 27G Soft needle: 25G |
Hard needle: 27G Soft needle: 25G |
Two 27G |
Table 1: CCBio’s injector platform products.
Cartridge-Based Design
CCBio also provides an array of cartridge-based injection pens (Figure 3). The primary container format for these devices is a 3 mL glass cartridge (Table 2), which provides a reliable and versatile solution for storing and delivering injectable drugs via the subcutaneous administration route.
| Property | Kratos | Aurora | Castor | Pollux |
| Device Type | Manual pen | Spring-assisted pen | Manual pen | Manual pen |
| Usage | Fixed 0.08 mL dose; disposable | Mutidose; disposable | Mutidose; disposable | Mutidose; disposable |
| Dose Volume | 0.08 mL | 0.01–0.8 mL | 0.01–0.6 mL | 0.01–0.6 mL |
Table 2: CCBio’s cartridge-based design injector platform products.
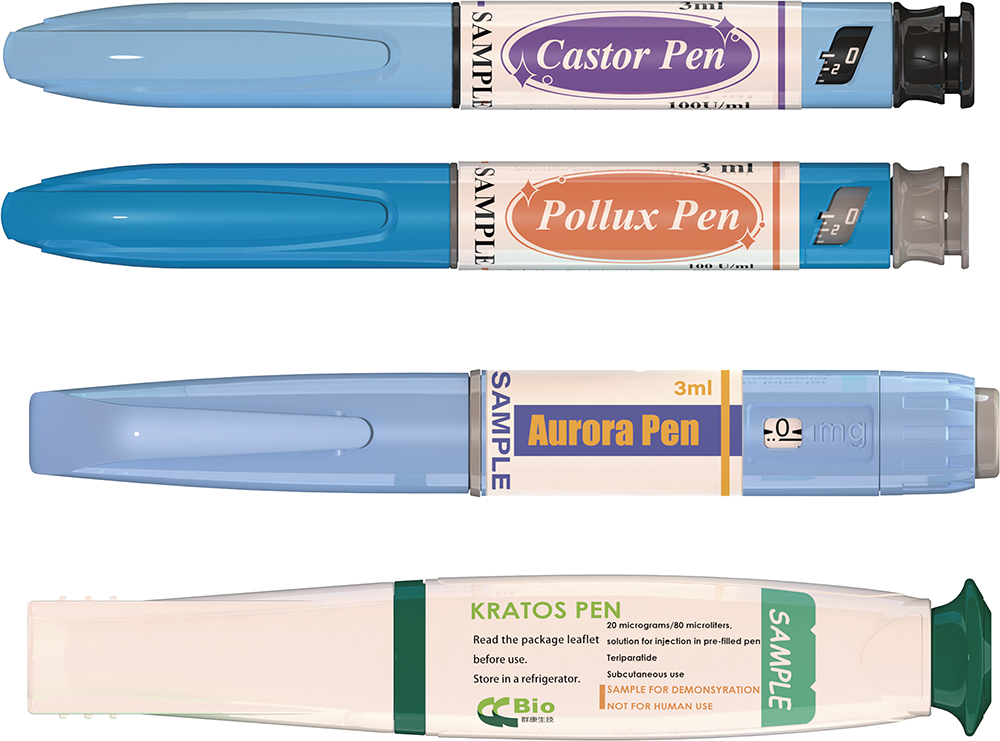
Figure 3: CCBio’s cartridge-based pen injectors.
PFS-Based Design
Completing its product portfolio, CCBio offers two single-use autoinjectors (Figure 4). The primary container format for these devices is either a 1 or a 2.25 mL PFS (Table 3), which provides a reliable and versatile solution for storing and delivering injectable drugs via the subcutaneous administration route.
| Property | Salus | Proserpine |
| Device Type | 27–29G autoinjector | 27–29G autoinjector |
| Usage | Single dose; disposable | Single dose; disposable |
| Dose Volume | 0.5–0.75 mL | 0.3–2 mL |
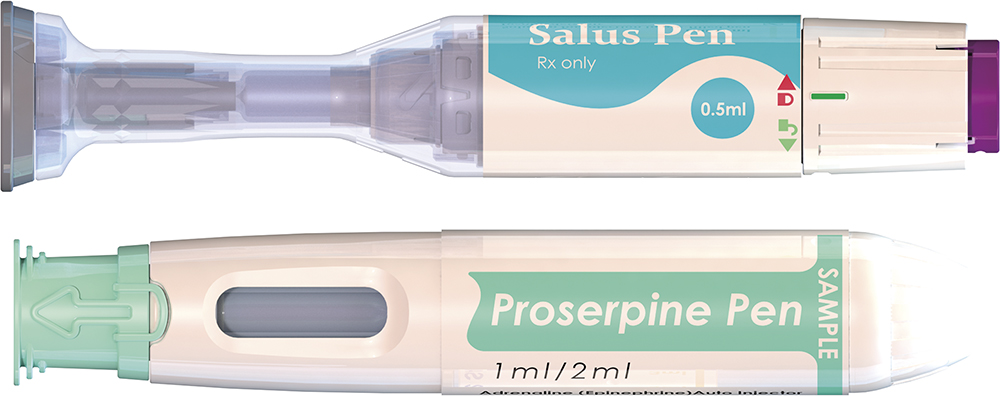
Figure 4: CCBio’s PFS-based pen injectors.
Table 3: CCBio’s PFS-based injector platform products.
CCBIO’S AUTOMATED ASSEMBLY LINE FOR SELF-INJECTION PRODUCTS
CCBio is proud to be a fully Taiwan-based company, taking full advantage of Taiwan’s strong advantages in semiconductors, metal processing and automation. The company offers a fully integrated service, applying top-tier craftsmanship to its products and manufacturing processes (Figure 5). As a trusted supplier, CCBio ensures that its customers receive comprehensive, high-quality solutions tailored to their requirements.

Figure 5: CCBio’s automated assembly line.
REFERENCE
- Molina JT, Robledillo JCR, Ruiz NC, “Potential Benefits of the Self-Administration of Subcutaneous Methotrexate with Autoinjector Devices for Patients: A Review”. Drug Healthc Patient Saf, 2021, Vol 13, pp 81–94.

